Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Mechanisms of Oxytocin and Serotonin Interaction in Non-Human Primates and Patients with Autism Arthur Lefevre
Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism Arthur Lefevre To cite this version: Arthur Lefevre. Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism. Neuroscience. Université de Lyon, 2016. English. NNT : 2016LYSE1323. tel-01508642 HAL Id: tel-01508642 https://tel.archives-ouvertes.fr/tel-01508642 Submitted on 14 Apr 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N°d’ordre NNT : xxx THESE de DOCTORAT DE L’UNIVERSITE DE LYON opérée au sein de l’Université Claude Bernard Lyon 1 Ecole Doctorale 476 (Neuroscience et Cognition) Spécialité de doctorat : Discipline : Neurosciences Soutenue publiquement le 13 Décembre 2016, par : Arthur LEFEVRE __________________________________________ Neural mechanisms of oxytocin and serotonin interaction in non- human primates and patients with autism __________________________________________ Thèse dirigée par Angela Sirigu (DRCE, CNRS) Devant le jury composé de : GERVAIS Rémi, Président, (Professeur à l’Université Lyon 1 Claude Bernard) CHINI BICE, Rapporteur, (Senior researcher at the Institute of Neuroscience, Milan) CHAKRABARTI Bhismadev, Rapporteur, (Associate Professor at university of Reading and Senior researcher at Cambridge university) KRAUS Christoph, Examinateur, (Senior researcher at the Medical university of Vienna) SIRIGU Angela, Directrice de thèse, (DRCE, Université de Lyon, CNRS) 1 UNIVERSITE CLAUDE BERNARD - LYON 1 Président de l’Université M. -

Dopamine and Serotonin Metabolism in Parkinsonian Models
Dopamine and Serotonin Metabolism in Parkinsonian Models Carmen de la Fuente Barrigon UCL Great Ormond Street Institute of Child Health Thesis submitted for the degree of Doctor of Philosophy (PhD) awarded by University College London (UCL). Funded by Marie Skłodowska-Curie Actions of the European Union’s Seventh Framework Programme (FP7). APRIL 2018 1 I, Carmen de la Fuente Barrigon confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed ………………………………………….. Date ……………………………………………… 3 Dedico esta tesis a los pilares de mi vida. A mis padres, Tomás y Marisa. A mi hermana, Paloma. A mi amor, Francesco. Por vuestros sacrificios, paciencia, amor y apoyo incondicionales que me hacen ser quien soy hoy. 5 Abstract Parkinson’s disease (PD) is a neurodegenerative disorder caused by loss of dopaminergic neurons in the substantia nigra. Different pathogenic mechanisms have been implicated, including loss of mitochondrial complex I function and dysfunction of lysosomal glucocerebrosidase (GBA1) (Neumann et al., 2009; Schapira et al., 1990). Also, it has been hypothesised that serotonin metabolism could be affected in these patients due to the number of enzymes shared by both pathways (Albizu et al., 2011). This thesis considers the potential involvement of complex I and GBA1 in PD using HPLC analysis of changes in the extracellular levels of the metabolites of dopamine and serotonin, and the expression and activity of the enzymes of the dopamine pathway. Using SH-SY5Y cells, complex I deficiency was modelled using rotenone, and GBA1 deficiency was modelled using conduritol B epoxide (CBE). -

Activation of the Dopaminergic Pathway from VTA to the Medial
RESEARCH ARTICLE Activation of the dopaminergic pathway from VTA to the medial olfactory tubercle generates odor-preference and reward Zhijian Zhang1,2†, Qing Liu1†, Pengjie Wen1, Jiaozhen Zhang1, Xiaoping Rao1, Ziming Zhou3, Hongruo Zhang3, Xiaobin He1, Juan Li1, Zheng Zhou4, Xiaoran Xu3, Xueyi Zhang3, Rui Luo3, Guanghui Lv2, Haohong Li2, Pei Cao1, Liping Wang4, Fuqiang Xu1,2* 1Center for Brain Science, Key Laboratory of Magnetic Resonance in Biological Systems, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan, China; 2Wuhan National Laboratory for Optoelectronics, Wuhan, China; 3College of Life Sciences, Wuhan University, Wuhan, China; 4Shenzhen Key Lab of Neuropsychiatric Modulation and Collaborative Innovation Center for Brain Science, CAS Center for Excellence in Brain Science, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China Abstract Odor-preferences are usually influenced by life experiences. However, the neural circuit mechanisms remain unclear. The medial olfactory tubercle (mOT) is involved in both reward and olfaction, whereas the ventral tegmental area (VTA) dopaminergic (DAergic) neurons are considered to be engaged in reward and motivation. Here, we found that the VTA (DAergic)-mOT pathway could be activated by different types of naturalistic rewards as well as odors in DAT-cre mice. Optogenetic activation of the VTA-mOT DAergic fibers was able to elicit preferences for space, location and neutral odor, while pharmacological blockade of the dopamine receptors in the *For correspondence: mOT fully prevented the odor-preference formation. Furthermore, inactivation of the mOT- [email protected] projecting VTA DAergic neurons eliminated the previously formed odor-preference and strongly †These authors contributed affected the Go-no go learning efficiency. -

Long-Range Gabaergic Projections Contribute to Cortical Feedback
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.19.423599; this version posted December 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Long-range GABAergic projections contribute to cortical feedback control of sensory processing. Camille Mazo1,2, *, Soham Saha1, Antoine Nissant1, Enzo Peroni1, Pierre-Marie Lledo1, # and Gabriel Lepousez1,#,* 1 Laboratory for Perception and Memory, Institut Pasteur, F-75015 Paris, France; Centre National de la Recherche Scientifique (CNRS), Unité Mixte de Recherche (UMR-3571), F-75015 Paris, France. * Corresponding authors to whom correspondence should be addressed: Laboratory for Perception and Memory, Institut Pasteur, 25 rue du Dr. Roux, 75 724 Paris Cedex 15, France. Tel: (33) 1 45 68 95 23 E-mail: [email protected] E-mail: [email protected] # Jointly supervised this work 2 now at Champalimaud Research, Champalimaud Center for the Unknown, Lisbon, Portugal Keywords: Sensory circuits, Top-down, Inhibitory, Centrifugal, Olfactory system, Barrel cortex 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.12.19.423599; this version posted December 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Abstract In sensory systems, cortical areas send excitatory projections back to subcortical areas to dynamically adjust sensory processing. -

Estrogen Receptors Α, Β and GPER in the CNS and Trigeminal System - Molecular and Functional Aspects Karin Warfvinge1,2, Diana N
Warfvinge et al. The Journal of Headache and Pain (2020) 21:131 The Journal of Headache https://doi.org/10.1186/s10194-020-01197-0 and Pain RESEARCH ARTICLE Open Access Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects Karin Warfvinge1,2, Diana N. Krause2,3†, Aida Maddahi1†, Jacob C. A. Edvinsson1,4, Lars Edvinsson1,2,5* and Kristian A. Haanes1 Abstract Background: Migraine occurs 2–3 times more often in females than in males and is in many females associated with the onset of menstruation. The steroid hormone, 17β-estradiol (estrogen, E2), exerts its effects by binding and activating several estrogen receptors (ERs). Calcitonin gene-related peptide (CGRP) has a strong position in migraine pathophysiology, and interaction with CGRP has resulted in several successful drugs for acute and prophylactic treatment of migraine, effective in all age groups and in both sexes. Methods: Immunohistochemistry was used for detection and localization of proteins, release of CGRP and PACAP investigated by ELISA and myography/perfusion arteriography was performed on rat and human arterial segments. Results: ERα was found throughout the whole brain, and in several migraine related structures. ERβ was mainly found in the hippocampus and the cerebellum. In trigeminal ganglion (TG), ERα was found in the nuclei of neurons; these neurons expressed CGRP or the CGRP receptor in the cytoplasm. G-protein ER (GPER) was observed in the cell membrane and cytoplasm in most TG neurons. We compared TG from males and females, and females expressed more ER receptors. For neuropeptide release, the only observable difference was a baseline CGRP release being higher in the pro-estrous state as compared to estrous state. -

Investigations Into Neuronal Cilia Utilizing Mouse Models
INVESTIGATIONS INTO NEURONAL CILIA UTILIZING MOUSE MODELS OF BARDET-BIEDL SYNDROME Dissertation Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Nicolas F. Berbari, BS ***** The Ohio State University 2008 Dissertation Committee: Approved by: Kirk Mykytyn, PhD, Adviser Virginia Sanders, PhD __________________________________________ Georgia Bishop, PhD Adviser Michael Robinson, PhD Integrated Biomedical Sciences Graduate Program ABSTRACT Cilia are hair-like microtubule based cellular appendages that extend 5-30 microns from the surface of most vertebrate cells. Since their initial discovery over a hundred years ago, cilia have been of interest to microbiologists and others studying the dynamics and physiological relevance of their motility. The more recent realization that immotile or primary cilia dysfunction is the basis of several human genetic disorders and diseases has brought the efforts of the biomedical research establishment to bear on this long overlooked and underappreciated organelle. Several human genetic disorders caused by cilia defects have been identified, and include Bardet-Biedl syndrome, Joubert syndrome, Meckel-Gruber syndrome, Alstrom syndrome and orofaciodigital syndrome. One theme of these disorders is their multitude of clinical features such as blindness, cystic kidneys, cognitive deficits and obesity. The fact that many of these cilia disorders present with several features may be due to the ubiquitous nature of the primary cilium and their unrecognized roles in most tissues and cell types. The lack of known function for most primary cilia is no more apparent than in the central nervous system. While it has been known for some time that neurons throughout the brain have primary cilia, their functions remain unknown. -

Does the Kappa Opioid Receptor System Contribute to Pain Aversion?
UC Irvine UC Irvine Previously Published Works Title Does the kappa opioid receptor system contribute to pain aversion? Permalink https://escholarship.org/uc/item/8gx6n97q Authors Cahill, Catherine M Taylor, Anna MW Cook, Christopher et al. Publication Date 2014 DOI 10.3389/fphar.2014.00253 Peer reviewed eScholarship.org Powered by the California Digital Library University of California REVIEW ARTICLE published: 17 November 2014 doi: 10.3389/fphar.2014.00253 Does the kappa opioid receptor system contribute to pain aversion? Catherine M. Cahill 1,2,3 *, Anna M. W. Taylor1,4 , Christopher Cook1,2 , Edmund Ong1,3 , Jose A. Morón5 and Christopher J. Evans 4 1 Department of Anesthesiology and Perioperative Care, University of California Irvine, Irvine, CA, USA 2 Department of Pharmacology, University of California Irvine, Irvine, CA, USA 3 Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, ON, Canada 4 Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, USA 5 Department of Anesthesiology, Columbia University Medical Center, New York, NY, USA Edited by: The kappa opioid receptor (KOR) and the endogenous peptide-ligand dynorphin have Dominique Massotte, Institut des received significant attention due the involvement in mediating a variety of behavioral Neurosciences Cellulaires et Intégratives, France and neurophysiological responses, including opposing the rewarding properties of drugs of abuse including opioids. Accumulating evidence indicates this system is involved in Reviewed by: Lynn G. Kirby, University of regulating states of motivation and emotion. Acute activation of the KOR produces an Pennsylvania, USA increase in motivational behavior to escape a threat, however, KOR activation associated Clifford John Woolf, Boston Children’s with chronic stress leads to the expression of symptoms indicative of mood disorders. -

A Cortical Pathway Modulates Sensory Input Into the Olfactory Striatum 3 4 5 Kate A
bioRxiv preprint doi: https://doi.org/10.1101/235291; this version posted December 16, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 2 A cortical pathway modulates sensory input into the olfactory striatum 3 4 5 Kate A. White1,2,3, Yun-Feng Zhang4, Zhijian Zhang5, Janardhan P. Bhattarai4, Andrew 6 H. Moberly4, Estelle in ‘t Zandt1,2, Huijie Mi6, Xianglian Jia7, Marc V. Fuccillo4, Fuqiang 7 Xu5, Minghong Ma4, Daniel W. Wesson1,2,3* 8 9 1Department of Pharmacology & Therapeutics 10 2Center for Smell and Taste 11 University of Florida 12 1200 Newell Dr.; Gainesville, FL, 32610. U.S.A. 13 3Department of Neurosciences 14 Case Western Reserve University 15 2109 Adelbert Rd.; Cleveland, OH, 44106. U.S.A. 16 4Department of Neuroscience 17 University of Pennsylvania Perelman School of Medicine 18 211 CRB, 415 Curie Blvd; Philadelphia, PA, 19104. U.S.A 19 5Center for Brain Science 20 Wuhan Institute of Physics and Mathematics 21 Chinese Academy of Sciences 22 Wuhan 430071, China 23 6College of Life Sciences 24 Wuhan University 25 Wuhan 430072, China 26 7Shenzhen Institutes of Advanced Technology 27 Chinese Academy of Sciences 28 Shenzhen 518055, China 29 30 *corresponding author; [email protected] 31 RUNNING HEAD: Olfactory striatum input 32 33 Author Contributions: Conceptualization: K.A.W. and D.W.W.; Methodology: K.A.W., Z.Z., F.X., 34 M.M., and D.W.W.; Investigation: K.A.W., Y-F.Z., Z.Z., J.P.B., A.H.M., E.I.Z., H.M., and X.J.; 35 Resources: M.V.F.; Writing – Original Draft: K.A.W., Z.Z., M.M., and D.W.W.; Writing – Review & 36 Editing: all authors; Visualization: K.A.W., Z.Z., Y-F.Z., J.P.B., D.W.W.; Supervision: F.X., M.M., 37 and D.W.W.; Funding Acquisition: K.A.W., F.X., M.M., and D.W.W. -

Neurophysiological and Neurohumoral Changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (Nac)
From the Department of Neurosurgery of the University of Luebeck Director: Prof. Dr. med. Volker Tronnier Neurophysiological and neurohumoral changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (NAc) Dissertation for Fulfillment of Requirements for the Doctoral Degree of the University of Luebeck from the Department of Natural Sciences Submitted by Ramya Varatharajan Thirugnanasambantham Born in Veeramanipatti-TN, India Luebeck, 2015 I Ramya Varatharajan Thirugnanasambantham Department of Neurosurgery University of Luebeck Ratzeburger allee 160 23562, Luebeck Germany First referee: Prof. Dr. Volker Tronnier Second referee: Prof. Dr. Thomas Martinetz Date of oral examination: 23.09.2015 Approved for printing, Luebeck: 30.09.2015 II Acknowledgements The hard work of so many people is involved in successful completion of this thesis, that a genuine list is virtually impossible. I convey my thanks and gratitude to Prof. Dr. med. Volker Tronnier, Department of Neurosurgery, for providing this opportunity to work under his guidance with this interesting topic. I express my deepest gratitude to Prof. Dr. med. Andreas Moser and Prof. Dr. rer. nat. Ulrich G. Hofmann for providing Laboratory Infrastructure, financial and moral support. Beyond scientific supervision, I was lucky to have the people from liquorlab, guiding me through all matters during my stay in Luebeck. I am thankful to Katharina Schnackenberg whose excellent experience in the laboratory and generous practical help was essential for the successful work at the lab. Thanks to Karin Wiegers, Marie Luis Reher and Gisa Brunk for being like friends and taking good care of me. I am very much thankful to Krishnakumar, Kevin Joseph, Sonya Neto, Mehrnaz Hazrati, Yijing Xie and Susanne Loeffler for their support, encouragement and timely suggestions. -

(12) United States Patent (10) Patent No.: US 6,969,702 B2 Bertilsson Et Al
USOO6969702B2 (12) United States Patent (10) Patent No.: US 6,969,702 B2 Bertilsson et al. (45) Date of Patent: Nov. 29, 2005 (54) COMPOUNDS AND METHODS FOR OTHER PUBLICATIONS INCREASING NEUROGENESIS Jackowski, "Neural injury repair: hope for the future as (75) Inventors: Göran Bertilsson, Västerhaninge (SE); barriers to effective CNS regeneration become clearer,' Rikard Erlandsson, Sundyberg (SE); British Journal of Neurosurgery, (1995), 9, p. 303-317.* Jonas Frisen, Stockholm (SE); Anders Asanuma et al. (1996). Mol. Brain Res. 41: 210-215. Haegerstrand, Danderyd (SE); Jessica Cameron and McKay (1998). Current Opinion in Neurobiol. Heidrich, Arsta (SE); Nina Hellström, 8: 677-680. Södertälje (SE); Johan Haggblad, Cassidy and Frisen (2001). Nature 412: 690-691. Västgötagränd (SE); Katarina Jansson, Dinter et al. (1997). J. Mol. Med. 75: 95-102. Johanneshov (SE); Jarkko Kortesmaa, D'Sa and Duman (2002). Bipolar Disorders 4: 183–194. Stockholm (SE); Per Lindquist, Duman et al. (2001). J. Pharmacol. and Ex. Therapeutics Bromma (SE); Hanna Lundh, Solna 299: 4O1-4O7. (SE); Jacqueline McGuire, Stockholm Duman et al. (2001). Neuropsychopharmacol. 25: 836-844. (SE); Alex Mercer, Bromma (SE); Duprat et al. (2000). Mol. Pharmacol. 57: 906–912. Karl Nyberg, Uppsala (SE); Amina Hallbergson et al. (2003). J. Clinical Investigation 112: Ossoinak, Stockholm (SE); Cesare 1128-1133. Patrone, Hägersten (SE); Harriet Hartikka et al. (1992). J. Neuroscience Res. 32: 190–201. Iona et al. (1998). Mol. Pharmacol. 53: 23-32. Rönnholm, Trångsund (SE); Lilian Kim et al. (2000). Society for Neuroscience 26: 2316, Wikström, Spånga (SE); Olof Abstract No. 868.2. Zachrisson, Spånga (SE) Malberg et al. (2000). J. -
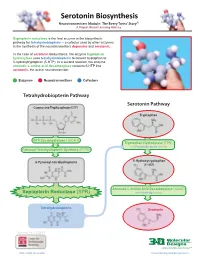
Serotonin Biosynthesis Neurotransmitters Module: the Beery Twins’ Story© a Project-Based Learning Activity
Serotonin Biosynthesis Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity Sepiapterin reductase is the final enzyme in the biosynthetic pathway for tetrahydrobiopterin – a cofactor used by other enzymes in the synthesis of the neurotransmitters dopamine and serotonin. In the case of serotonin biosynthesis, the enzyme tryptophan hydroxylase uses tetrahydrobiopterin to convert tryptophan to 5-hydroxytryptophan (5-HTP). In a second reaction, the enzyme aromatic L-amino acid decarboxylase converts 5-HTP into serotonin, the active neurotransmitter. Enzymes Neurotransmitters Cofactors Tetrahydrobiopterin Pathway Serotonin Pathway Guanosine Triphosphate (GTP) Tryptophan GTP Cyclohydrolase I (GCH1) Tryptophan Hydroxylase (TPH) with Tetrahydrobiopterin Cofactor Pyruvoyl-Tetrahydropterin Synthase (PTPS) 6-Pyruvoyl-tetrahydropterin 5-Hydroxytryptophan (5-HTP) Aromatic L-Amino Acid Decarboxylase (AAAD) Sepiapterin Reductase (SPR) with Vitamin B6 Cofactor Tetrahydrobiopterin Serotonin Version 1.4 -12/2015 ...where molecules become real TM http://cbm.msoe.edu www.3dmoleculardesigns.com Serotonin Biosynthesis Model Guide Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity TPH AAAD Tryptophan 5-Hydroxytryptophan Serotonin Tryptophan (Trp or W) is one of the 20 standard 5-Hydroxytryptophan, an intermediate The nal step in the serotonin biosynthesis amino acids and is an essential amino acid that molecule in the serotonin biosynthesis pathway requires the removal of the cannot be synthesized by the human body. pathway, is formed by the addition of a carboxylic acid group (COOH) from the Tryptophan is composed of the standard amino hydroxyl (OH) group to the fth carbon of the backbone of 5-hydroxytryptophan to form acid backbone with an indole ring side chain. indole ring of tryptophan. -

Precise Detection of Direct Glomerular Input Duration by the Olfactory Bulb
16058 • The Journal of Neuroscience, November 26, 2014 • 34(48):16058–16064 Systems/Circuits Precise Detection of Direct Glomerular Input Duration by the Olfactory Bulb Anan Li,1,2 David H. Gire,3 Thomas Bozza,4 and Diego Restrepo1 1Department of Cell and Developmental Biology, Neuroscience Program and Rocky Mountain Taste and Smell Center, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, 2Wuhan Institute of Physics and Mathematics, The Chinese Academy of Sciences/State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan, China 430071, 3Department of Molecular and Cellular Biology, and Center for Brain Science, Harvard University, Cambridge, Massachusetts 02138, and 4Department of Neurobiology, Northwestern University, Evanston, Illinois 60208 Sensory neuron input to the olfactory bulb (OB) was activated precisely for different durations with blue light in mice expressing channelrhodopsin-2 in olfactory sensory neurons. Behaviorally the mice discriminated differences of 10 ms in duration of direct glomer- ular activation. In addition, a subset of mitral/tufted cells in the OB of awake mice responded tonically therefore conveying information on stimulus duration. Our study provides evidence that duration of the input to glomeruli not synchronized to sniffing is detected. This potent cue may be used to obtain information on puffs in odor plumes. Key words: olfactory; puffs Introduction sniffing (Wachowiak, 2011) and because of complex interactions Duration is an important parameter of sensory stimuli exten- between odorants and the epithelium (Schoenfeld and Cleland, sively studied in both the visual and auditory systems (B.G. Cle- 2005; Scott et al., 2014). Here we precisely controlled sensory land et al., 1971; Ikeda and Wright, 1974; Ehrlich et al., 1997; input to the olfactory bulb (OB) with blue light using a strain of Klink and Klump, 2004).