Clotting and Fibrinolysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isoelectric Focussing of Human Thyroxine Binding Globulin (Thyropexin) and Human Prealbumin (Transthyretin)
Luckenbach et al.: Isoelectric focussing of thyropexin and transthyretin 387 Eur. J. Clin. Chem. Clin. Biochem. Vol. 30, 1992, pp. 387-390 © 1992 Walter de Gruyter & Co. Berlin · New York Isoelectric Focussing of Human Thyroxine Binding Globulin (Thyropexin) and Human Prealbumin (Transthyretin) By Christine Luckenbach^ R. Wahl2 and E. Kailee2 1 Institut fur Anthropologie und Humangenetik 2 Medizinische Klinik und Poliklinik, Abteilung IV Eberhard-Karls- Universität Tübingen (Received November 7, 1991/Aprü 22, 1992) Summary: Two batches of the highly purified thyroid hormone-binding plasma proteins, human thyropexin and transthyretin, which were prepared in gram quantities for use in animal experiments, were subjected to analysis by isoelectric focussing. Under these conditions, it was observed that human transthyretin was composed of two components. This was presumably due to the use of 8 mol/1 urea. The preparations of both human transthyretin and human thyropexin contained some products of decomposition which probably arose in the course of the purification processes and, in addition, possibly also contained some normal genetic variants of human thyropexin. In spite of the alterations, both protein preparations largely retained their thyroid hormone-binding capacity, which is essential for in vivo studies on the re-entry of thyroid hormones from the extravascular space into the circulation. For therapeutic use in thyrotoxicosis, human transthyretin seems to be preferable to human thyropexin. Introduction The main thyroid hormone-binding plasma proteins severe thyrotoxicosis in emergencies: The concentra- in humans are thyropexin (1) ("TGB", human thy- tion of both T4 and T3 in the plasma can be signifi- roxine binding inter-alpha globulin (2))1) and trans- cantly enhanced by i.v. -

Urokinase, a Promising Candidate for Fibrinolytic Therapy for Intracerebral Hemorrhage
LABORATORY INVESTIGATION J Neurosurg 126:548–557, 2017 Urokinase, a promising candidate for fibrinolytic therapy for intracerebral hemorrhage *Qiang Tan, MD,1 Qianwei Chen, MD1 Yin Niu, MD,1 Zhou Feng, MD,1 Lin Li, MD,1 Yihao Tao, MD,1 Jun Tang, MD,1 Liming Yang, MD,1 Jing Guo, MD,2 Hua Feng, MD, PhD,1 Gang Zhu, MD, PhD,1 and Zhi Chen, MD, PhD1 1Department of Neurosurgery, Southwest Hospital, Third Military Medical University, Chongqing; and 2Department of Neurosurgery, 211st Hospital of PLA, Harbin, People’s Republic of China OBJECTIVE Intracerebral hemorrhage (ICH) is associated with a high rate of mortality and severe disability, while fi- brinolysis for ICH evacuation is a possible treatment. However, reported adverse effects can counteract the benefits of fibrinolysis and limit the use of tissue-type plasminogen activator (tPA). Identifying appropriate fibrinolytics is still needed. Therefore, the authors here compared the use of urokinase-type plasminogen activator (uPA), an alternate thrombolytic, with that of tPA in a preclinical study. METHODS Intracerebral hemorrhage was induced in adult male Sprague-Dawley rats by injecting autologous blood into the caudate, followed by intraclot fibrinolysis without drainage. Rats were randomized to receive uPA, tPA, or saline within the clot. Hematoma and perihematomal edema, brain water content, Evans blue fluorescence and neurological scores, matrix metalloproteinases (MMPs), MMP mRNA, blood-brain barrier (BBB) tight junction proteins, and nuclear factor–κB (NF-κB) activation were measured to evaluate the effects of these 2 drugs in ICH. RESULTS In comparison with tPA, uPA better ameliorated brain edema and promoted an improved outcome after ICH. -

Fibrinolysis and Anticoagulant Potential of a Metallo Protease Produced by Bacillus Subtilis K42
Fibrinolysis and anticoagulant potential of a metallo protease produced by Bacillus subtilis K42 WESAM AHASSANEIN, ESSAM KOTB*, NADIA MAWNY and YEHIA AEL-ZAWAHRY Department of Microbiology, Faculty of Science, Zagazig University, Zagazig, Egypt 44519 *Corresponding author (Email, [email protected]) In this study, a potent fibrinolytic enzyme-producing bacterium was isolated from soybean flour and identified as Bacillus subtilis K42 and assayed in vitro for its thrombolytic potential. The molecular weight of the purified enzyme was 20.5 kDa and purification increased its specific activity 390-fold with a recovery of 14%. Maximal activity was attained at a temperature of 40°C (stable up to 65°C) and pH of 9.4 (range: 6.5–10.5). The enzyme retained up to 80% of its original activity after pre-incubation for a month at 4°C with organic solvents such as diethyl ether (DE), toluene (TO), acetonitrile (AN), butanol (BU), ethyl acetate (EA), ethanol (ET), acetone (AC), methanol (ME), isopropanol (IP), diisopropyl fluorophosphate (DFP), tosyl-lysyl-chloromethylketose (TLCK), tosyl-phenylalanyl chloromethylketose (TPCK), phenylmethylsulfonylfluoride (PMSF) and soybean trypsin inhibitor (SBTI). Aprotinin had little effect on this activity. The presence of ethylene diaminetetraacetic acid (EDTA), a metal-chelating agent and two metallo protease inhibitors, 2,2′-bipyridine and o-phenanthroline, repressed the enzymatic activity significantly. This, however, could be restored by adding Co2+ to the medium. The clotting time of human blood serum in the presence of this enzyme reached a relative PTT of 241.7% with a 3.4-fold increase, suggesting that this enzyme could be an effective antithrombotic agent. -

The Central Role of Fibrinolytic Response in COVID-19—A Hematologist’S Perspective
International Journal of Molecular Sciences Review The Central Role of Fibrinolytic Response in COVID-19—A Hematologist’s Perspective Hau C. Kwaan 1,* and Paul F. Lindholm 2 1 Division of Hematology/Oncology, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA 2 Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] * Correspondence: [email protected] Abstract: The novel coronavirus disease (COVID-19) has many characteristics common to those in two other coronavirus acute respiratory diseases, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). They are all highly contagious and have severe pulmonary complications. Clinically, patients with COVID-19 run a rapidly progressive course of an acute respiratory tract infection with fever, sore throat, cough, headache and fatigue, complicated by severe pneumonia often leading to acute respiratory distress syndrome (ARDS). The infection also involves other organs throughout the body. In all three viral illnesses, the fibrinolytic system plays an active role in each phase of the pathogenesis. During transmission, the renin-aldosterone- angiotensin-system (RAAS) is involved with the spike protein of SARS-CoV-2, attaching to its natural receptor angiotensin-converting enzyme 2 (ACE 2) in host cells. Both tissue plasminogen activator (tPA) and plasminogen activator inhibitor 1 (PAI-1) are closely linked to the RAAS. In lesions in the lung, kidney and other organs, the two plasminogen activators urokinase-type plasminogen activator (uPA) and tissue plasminogen activator (tPA), along with their inhibitor, plasminogen activator 1 (PAI-1), are involved. The altered fibrinolytic balance enables the development of a hypercoagulable Citation: Kwaan, H.C.; Lindholm, state. -

Anticoagulant Drugs
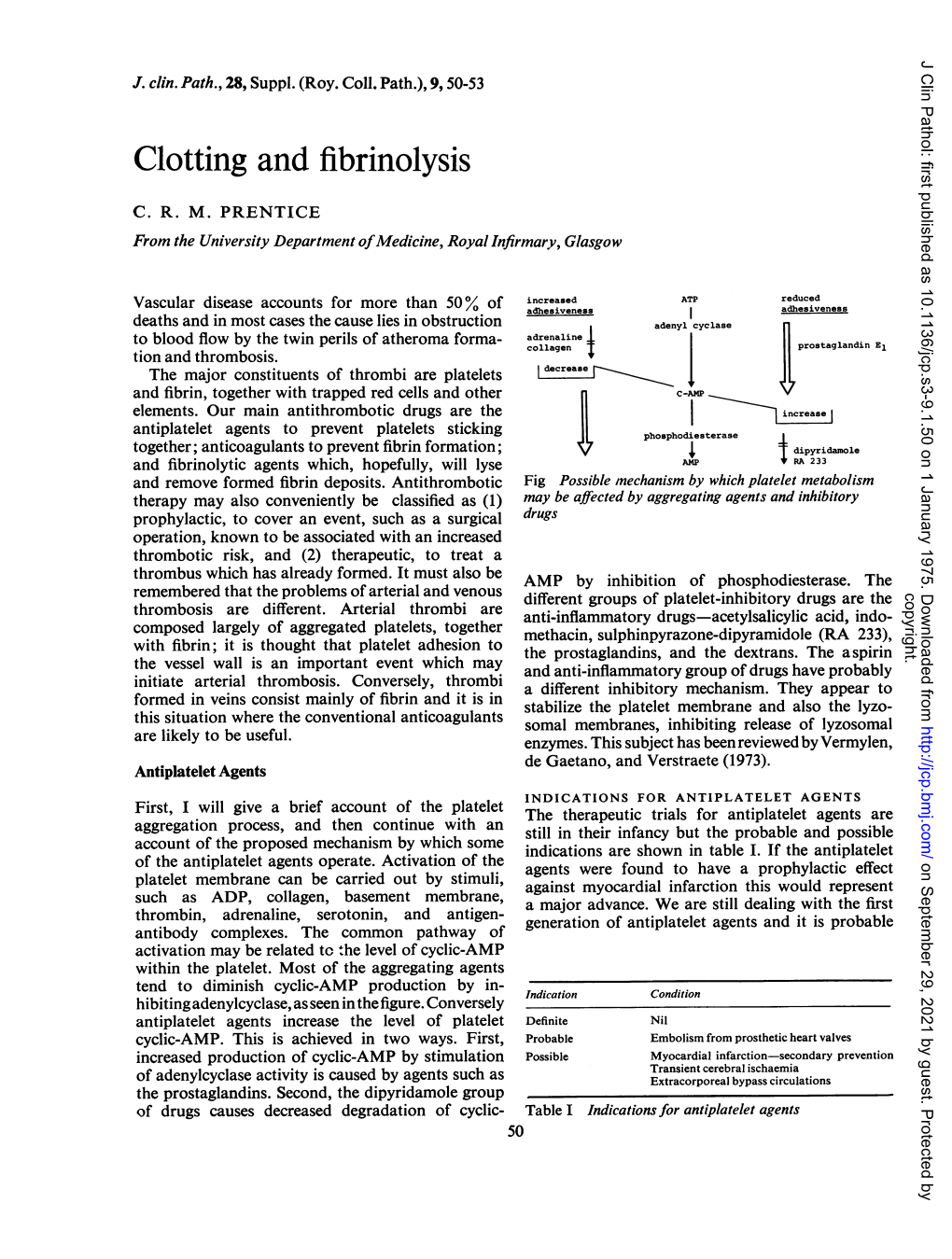
Anticoagulant drugs Objectives: Introduction about coagulation cascade. Classify drugs acting as anticoagulants. Elaborate on their mechanism of action, correlating with that methods of monitoring. Contrast the limitations and benefits of injectable anticoagulants in clinical settings. Emphasis on the limitations of VKAs and on variable altering or modifying their response. Done by: Editing file Abdulaziz Alhammad, Ibrahim AlAsoos, Yousef Alsamil, Mohammed Abunayan, Sara Alkhalifah, Khalid Aburas, Atheer Alnashwan Revision: Jwaher Alharbi, Qusay Ajlan, Khalid Aburas, Atheer Alnashwan Mind Map Antiplatelet drugs Anticoagulants Fibrinolytic agents Drugs acting on Coagulation pathways Focus of the lecture Anticoagulants Parenteral Oral Anticoagulants Anticoagulants Vitamin K Direct Indirect antagonists Hirudin, Heparin and Warfarin Lepirudin Heparin related agents The most important slides are: 5, 6, 7, 8, 10 & 11 To understand better Definitions we need to understand: They prevent thrombus formation and extension by Anticoagulants inhibiting clotting factors (e.g. heparin, low molecular weight heparin, coumarins (warfarin) Antiplatelet They reduce the risk of clot formation by inhibiting drugs platelet functions (e.g. aspirin and ticlopidine). Fibrinolytic They dissolve thrombi that is already formed (e.g. agents streptokinase) Coagulation pathways: 6:27min 7:43min (thromboplastin) Endogenous inhibitors of coagulation: It’s a plasma protein that acts by inhibiting the Anti-thrombin III activated thrombin (factor IIa) and inhibits factor Xa, it is the site of action of heparin. Prostacyclin It is synthesized by endothelial cells and inhibits (prostaglandin I2) platelet aggregation. These are vitamin K dependent proteins that slow the Protein C and coagulation cascade by inactivating factor Va and VIIIa. protein S The site of action of warfarin Anticoagulants Parenteral Oral Anticoagulant Anticoagulant Act as thrombin Act as Vitamin K inhibitors either in: antagonist (e.g. -

Immune Proteins, Enzymes and Electrolytes in Human Peripheral Lymph W.L
156 Lymphology 11 (1978) 156-164 Immune Proteins, Enzymes and Electrolytes in Human Peripheral Lymph W.L. Olszewski, A. Engeset Laboratory for Haematology and Lymphology, Norsk Hydro's Institute for Cancer Research, The Norwegian Radium Hospital, Oslo, Norway, and The Department of Surgical Research andTransplantology, Medical Research Center, Polish Academy of Sciences, Warsaw, Poland Summary bility. To do this, a better knowledge of kinetics Values of various biochemical constituents ofleg of transport of protein and other plasma consti lymph in 27 normal men have been presented. Con tuents to the tissue space of normal humans centration of immunoglobulins, complement proteins, seems to be necessary. acute phase reactants, enzymes, electrolytes and oth· er small molecular weight substances were measured. In the present paper we summarize the results of our studies on concentration of various pro Lymph forms part of the interstitial fluid with teins, among them of immunoglobulins, com a chemical composition resembling that of plement, and acute phase reactants, as well as blood plasma. It seems to be almost identical enzymes and electrolytes in the leg prenodal with the mobile tissue fluid. The concentra lymph in 27 men, during normal daily activities tion of macromolecules in tissue fluid and and experiments increasing the capillary filtra lymph depends on the ultrastructure of blood tion rate. We also analyze factors which cause capillaries and physical forces govepting trans variations in the level of biochemical consti port of substances across the capillary wall, tuents of tissue fluid and lymph. mostly hydrostatic and osmotic pressure gradients. The capillary wall acts as a molecu Materials and methods lar filter restricting free flow of proteins from Studies were carried out on 27 healthy male blood to the tissue space. -

Blood Coagulation and Haemostasis: a Review*
BLOOD COAGULATION AND HAEMOSTASIS: A REVIEW* E. M. RODF_~QUE,M.D. AND J. E. WYNANDS, M.D., C.IVI.~ FnOM THE V~Y F_~aLY STUVmS of this subject, many controversies existed regard- ing the various factors and mechanisms involved in the dotting process. One simply has to review the current literature and note the complex, often variable terminology, and the differing opinions of several workers in this field to realize that many of the former controversies are still present. However, many facts have been firmly established. This review will deal primarily with these facts and will mention the disputed points only when they appear pertinent to a better under- standing of the problems of controlling haemorrhage. Various factors are involved in the haemostatic process; the established ones include (1) the extravascular tissues; (2) the vasculature itself, the size and type of vessel being important; (3) the number of functioning platelets, and (4) the plasma coagulation system,x,2 THE ErmAVASCtrLABTISSUES While the extravaseular tissues do not play a major role in the control of bleeding, yet "their integrity and/or the variations in the resistance they offer to escaping blood may determine the bleeding response of a particular part of the body after injury. The "black eye' is a well-known example."2 In other situations in which there is easy bruisability, as in the aged, in poor nutrition, in some women, and in disease states such as the Ehlers-Danlos syndrome, it is likely that poor extravascular support is a deciding or contributing factor. 2 THE V~CULATVBE Previous to the more advanced and tested knowledge of today, abnormal vascular function was implicated as the basic pathogenetic factor in a variety of bleeding disorders. -

ACTIVASE (Alteplase) for Injection, for Intravenous Use Initial U.S
Application 103172 This document contains: Label for ACTIVASE [Supplement 5203, Action Date 02/13/2015] Also available: Label for CATHFLO ACTIVASE [Supplement 5071, Action Date 01/04/2005] HIGHLIGHTS OF PRESCRIBING INFORMATION Acute Ischemic Stroke These highlights do not include all the information needed to use • Current intracranial hemorrhage. (4.1) ACTIVASE safely and effectively. See full prescribing information for • Subarachnoid hemorrhage. (4.1) ACTIVASE. Acute Myocardial Infarction or Pulmonary Embolism • History of recent stroke. (4.2) ACTIVASE (alteplase) for injection, for intravenous use Initial U.S. Approval: 1987 -----------------------WARNINGS AND PRECAUTIONS----------------------- • Increases the risk of bleeding. Avoid intramuscular injections. Monitor for ---------------------------INDICATIONS AND USAGE-------------------------- bleeding. If serious bleeding occurs, discontinue Activase. (5.1) Activase is a tissue plasminogen activator (tPA) indicated for the treatment of • Monitor patients during and for several hours after infusion for orolingual • Acute Ischemic Stroke (AIS). (1.1) angioedema. If angioedema develops, discontinue Activase. (5.2) • Acute Myocardial Infarction (AMI) to reduce mortality and incidence of • Cholesterol embolism has been reported rarely in patients treated with heart failure. (1.2) thrombolytic agents. (5.3) Limitation of Use in AMI: the risk of stroke may be greater than the benefit • Consider the risk of reembolization from the lysis of underlying deep in patients at low risk of death -

A First in Class Treatment for Thrombosis Prevention. a Phase I
Journal of Cardiology and Vascular Medicine Research Open Access A First in Class Treatment for Thrombosis Prevention. A Phase I study with CS1, a New Controlled Release Formulation of Sodium Valproate 1,2* 2 3 2 1,2 Niklas Bergh , Jan-Peter Idström , Henri Hansson , Jonas Faijerson-Säljö , Björn Dahlöf 1Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden 2 Cereno Scientific AB, Gothenburg, Sweden 3 Galenica AB, Malmö, Sweden *Corresponding author: Niklas Bergh, The Wallenberg Laboratory for Cardiovascular Research Sahlgrenska University Hospi- tal Bruna Stråket 16, 413 45 Göteborg, Tel: +46 31 3421000; E-Mail: [email protected] Received Date: June 11, 2019 Accepted Date: July 25, 2019 Published Date: July 27, 2019 Citation: Niklas Bergh (2019) A First in Class Treatment for Thrombosis Prevention? A Phase I Study With Cs1, a New Con- trolled Release Formulation of Sodium Valproate. J Cardio Vasc Med 5: 1-12. Abstract Several lines of evidence indicate that improving fibrinolysis by valproic acid may be a fruitful strategy for throm- bosis prevention. This study investigated the safety, pharmacokinetics, and effect on biomarkers for thrombosis of CS1, a new advanced controlled release formulation of sodium valproate designed to produce optimum valproic acid concen- trations during the early morning hours, when concentrations of plasminogen activator inhibitor (PAI)-1 and the risk of thrombotic events is highest. Healthy volunteers (n=17) aged 40-65 years were randomized to receive single doses of one of three formulations of CS1 (FI, FII, and FIII). The CS1 FII formulation showed the most favorable pharmacokinetics and was chosen for multiple dosing. -

Investigating an Increase in Florida Manatee Mortalities Using a Proteomic Approach Rebecca Lazensky1,2, Cecilia Silva‑Sanchez3, Kevin J
www.nature.com/scientificreports OPEN Investigating an increase in Florida manatee mortalities using a proteomic approach Rebecca Lazensky1,2, Cecilia Silva‑Sanchez3, Kevin J. Kroll1, Marjorie Chow3, Sixue Chen3,4, Katie Tripp5, Michael T. Walsh2* & Nancy D. Denslow1,6* Two large‑scale Florida manatee (Trichechus manatus latirostris) mortality episodes were reported on separate coasts of Florida in 2013. The east coast mortality episode was associated with an unknown etiology in the Indian River Lagoon (IRL). The west coast mortality episode was attributed to a persistent Karenia brevis algal bloom or ‘red tide’ centered in Southwest Florida. Manatees from the IRL also had signs of cold stress. To investigate these two mortality episodes, two proteomic experiments were performed, using two‑dimensional diference in gel electrophoresis (2D‑DIGE) and isobaric tags for relative and absolute quantifcation (iTRAQ) LC–MS/MS. Manatees from the IRL displayed increased levels of several proteins in their serum samples compared to controls, including kininogen‑1 isoform 1, alpha‑1‑microglobulin/bikunen precursor, histidine‑rich glycoprotein, properdin, and complement C4‑A isoform 1. In the red tide group, the following proteins were increased: ceruloplasmin, pyruvate kinase isozymes M1/M2 isoform 3, angiotensinogen, complement C4‑A isoform 1, and complement C3. These proteins are associated with acute‑phase response, amyloid formation and accumulation, copper and iron homeostasis, the complement cascade pathway, and other important cellular functions. -

Intermittent Calf Compression: a Randomized Trial in Elective Hip
0276 0277 10:00 h 10:15 h PREVENTION OF DEEP VEIN THROMBOSIS (DVT) IN ABDOMINAL SUR INTERMITTENT CALF COMPRESSION: A RANDOMIZED TRIAL IN GERY. A PROSPECTIVE COMPARISON BETWEEN SODIUM PENTOSAN ELECTIVE HIP REPLACEMENT. A. Gall us and T. Darby, Departments of Haematology and Surgery, Flinders Medical POLYSULPHATE AND DEXTRAN 70. D. Bergqvist and H. Ljungner. Centre, Adelaide, South Australia. Department of Surgery, University of Lund, Malmö General We have evaluated venous thrombosis prevention with Hospital, Malmö, Sweden. intermittent calf compression in 78 patients aged over 50 years having elective hip replacement. 38 patients were randomly allotted to intermittent calf compression with a A prospective comparison has been made between PZ 68, "BOC-Roberts Venous Flow Stimulator", begun at the start of sodium pentosan polysulphate, and dextran 70 as prophylac surgery and continued for 7 days, while 40 patients had no tic agents against DVT after abdominal surgery. 109 prophylaxis. Age, weight, length of surgery, and other risk patients above 50 years of age were randomly allocated to factors were similar in the two groups. All patients had one of the two groups. The analysis after exclusions is ascending venography of the operated leg on the seventh day, based on 86 patients. PZ 68 was injected 75 mg s.c. twice or bilateral venography if routine 125I fibrinogen leg daily for one week and dextran 70 was infused 500 ml per- scanning and impedance plethysmography suggested thrombosis operatively, 500 ml immediately postoperatively and 500 on the unoperated side. ml on the first postoperative day. Diagnosis of DVT was made with the 125-I-fibrinogen test with phlébographie Venography showed thrombosis in 12/38 patients treated verification. -

(Tpa) As a Novel Treatment for Refractory COVID
Journal of Trauma and Acute Care Surgery, Publish Ahead of Print DOI: 10.1097/TA.0000000000002694 Is There a Role for Tissue Plasminogen Activator (tPA) as a Novel Treatment for Refractory COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS)? Hunter B. Moore1, Christopher D. Barrett2,3, Ernest E. Moore1,4, Robert C. McIntyre1, Peter K. Moore5, Daniel S. Talmor6, Frederick A. Moore7, and Michael B. Yaffe2,3,8 1 Department of Surgery, University of Colorado Denver, Denver, CO USA 2 Koch Institute for Integrative Cancer Research, Center for Precision Cancer Medicine, Departments of Biological Engineering and Biology, Massachusetts Institute of Technology, Cambridge MA, USA 3 Division of Acute Care Surgery, Trauma and Surgical Critical Care, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA USA 4 Ernest E Moore Shock Trauma Center at Denver Health, Department of Surgery, Denver, CO USA 5 Department of Medicine, University of Colorado Denver, Denver CO USA 6 Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA USA 7 Department of Surgery, University of Florida, Gainesville, FL USA 8 ACCEPTED To whom correspondence should be addressed: E-mail: [email protected], Ph: 617- 452-2103, Fax: 617-452-2978 1 This work was supported by NIH Grants UM1-HL120877 (EEM, MBY), F32-HL134244 (CDB), and L30-GM120751 (CDB); and DoD Peer Reviewed Medical Research Program, Contract Number W81XWH-16-1-0464 (MBY). Keywords: COVID-19; Acute Respiratory Distress Syndrome (ARDS); Tissue Plasminogen Activator (tPA); Pulmonary Failure; Fibrinolysis This is an open-access article distributed under the terms of the Creative Commons Attribution- Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited.