Origin and Consequences of Necroinflammation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
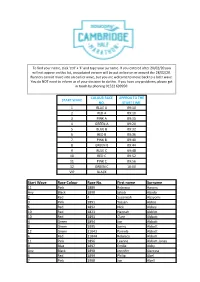
Start Wave Race Colour Race No. First Name Surname
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 20/02/20 you will not appear on this list, an updated version will be put online on or around the 28/02/20. Runners cannot move into an earlier wave, but you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. If you have any problems, please get in touch by phoning 01522 699950. COLOUR RACE APPROX TO THE START WAVE NO. START TIME 1 BLUE A 09:10 2 RED A 09:10 3 PINK A 09:15 4 GREEN A 09:20 5 BLUE B 09:32 6 RED B 09:36 7 PINK B 09:40 8 GREEN B 09:44 9 BLUE C 09:48 10 RED C 09:52 11 PINK C 09:56 12 GREEN C 10:00 VIP BLACK Start Wave Race Colour Race No. First name Surname 11 Pink 1889 Rebecca Aarons Any Black 1890 Jakob Abada 2 Red 4 Susannah Abayomi 3 Pink 1891 Yassen Abbas 6 Red 1892 Nick Abbey 10 Red 1823 Hannah Abblitt 10 Red 1893 Clare Abbott 4 Green 1894 Jon Abbott 8 Green 1895 Jonny Abbott 12 Green 11043 Pamela Abbott 6 Red 11044 Rebecca Abbott 11 Pink 1896 Leanne Abbott-Jones 9 Blue 1897 Emilie Abby Any Black 1898 Jennifer Abecina 6 Red 1899 Philip Abel 7 Pink 1900 Jon Abell 10 Red 600 Kirsty Aberdein 6 Red 11045 Andrew Abery Any Black 1901 Erwann ABIVEN 11 Pink 1902 marie joan ablat 8 Green 1903 Teresa Ablewhite 9 Blue 1904 Ahid Abood 6 Red 1905 Alvin Abraham 9 Blue 1906 Deborah Abraham 6 Red 1907 Sophie Abraham 1 Blue 11046 Mitchell Abrams 4 Green 1908 David Abreu 11 Pink 11047 Kathleen Abuda 10 Red 11048 Annalisa Accascina 4 Green 1909 Luis Acedo 10 Red 11049 Vikas Acharya 11 Pink 11050 Catriona Ackermann -

IN TAX LEADERS WOMEN in TAX LEADERS | 4 AMERICAS Latin America
WOMEN IN TAX LEADERS THECOMPREHENSIVEGUIDE TO THE WORLD’S LEADING FEMALE TAX ADVISERS SIXTH EDITION IN ASSOCIATION WITH PUBLISHED BY WWW.INTERNATIONALTAXREVIEW.COM Contents 2 Introduction and methodology 8 Bouverie Street, London EC4Y 8AX, UK AMERICAS Tel: +44 20 7779 8308 4 Latin America: 30 Costa Rica Fax: +44 20 7779 8500 regional interview 30 Curaçao 8 United States: 30 Guatemala Editor, World Tax and World TP regional interview 30 Honduras Jonathan Moore 19 Argentina 31 Mexico Researchers 20 Brazil 31 Panama Lovy Mazodila 24 Canada 31 Peru Annabelle Thorpe 29 Chile 32 United States Jason Howard 30 Colombia 41 Venezuela Production editor ASIA-PACIFIC João Fernandes 43 Asia-Pacific: regional 58 Malaysia interview 59 New Zealand Business development team 52 Australia 60 Philippines Margaret Varela-Christie 53 Cambodia 61 Singapore Raquel Ipo 54 China 61 South Korea Managing director, LMG Research 55 Hong Kong SAR 62 Taiwan Tom St. Denis 56 India 62 Thailand 58 Indonesia 62 Vietnam © Euromoney Trading Limited, 2020. The copyright of all 58 Japan editorial matter appearing in this Review is reserved by the publisher. EUROPE, MIDDLE EAST & AFRICA 64 Africa: regional 101 Lithuania No matter contained herein may be reproduced, duplicated interview 101 Luxembourg or copied by any means without the prior consent of the 68 Central Europe: 102 Malta: Q&A holder of the copyright, requests for which should be regional interview 105 Malta addressed to the publisher. Although Euromoney Trading 72 Northern & 107 Netherlands Limited has made every effort to ensure the accuracy of this Southern Europe: 110 Norway publication, neither it nor any contributor can accept any regional interview 111 Poland legal responsibility whatsoever for consequences that may 86 Austria 112 Portugal arise from errors or omissions, or any opinions or advice 87 Belgium 115 Qatar given. -

BOLETÍN OFICIAL DE LA PROVINCIA DE ALICANTE BUTLLETÍ OFICIAL PROVÍNCIA D'alacant Edita Excma
BOLETÍN OFICIAL DE LA PROVINCIA DE ALICANTE BUTLLETÍ OFICIAL PROVÍNCIA D'ALACANT edita excma. diputación provincial - alicante edita excma. diputació provincial - alacant miércoles, 3 de junio de 2009 dimecres, 3 de juny de 2009 Sumario Pág. Pág. Núm. Núm. ADMINISTRACIÓN CENTRAL: AYUNTAMIENTO ALICANTE. -LISTAS PROVISIONALES ASPIRANTES ADMITIDOS Y EXCLUIDOS INSPECCIÓN PROVINCIAL DE TRABAJO ALICANTE. VARIAS PLAZAS OFERTA DE EMPLEO PÚBLICO 2005 Y 2008 74 -NOTIFICACIÓN RESOLUCIÓN ACTAS DE INFRACCIÓN 3 AYUNTAMIENTO ALMORADÍ. INSTITUTO NACIONAL DE LA SEGURIDAD SOCIAL ALICANTE. -BAJAS DE OFICIO EN EL PADRON DE HABITANTES 107 -NOTIFICACIÓN PETICIÓN DE DOCUMENTACIÓN QUE ACREDITE -BAJAS DE OFICIO EN EL PADRON DE HABITANTES 107 SU IDENTIDAD 3 AYUNTAMIENTO BENISSA. JEFATURA PROVINCIAL DE TRÁFICO ALICANTE. -LISTA PROVISIONAL ADMITIDOS Y EXCLUIDOS Y TRIBUNAL -NOTIFICACIÓN DE RESOLUCIONES RECAIDAS EN EXPEDIENTES CALIFICADOR CONVOCATORIA TRES PLAZAS POLICÍA LOCAL 108 SANCIONADORES 3 AYUNTAMIENTO EL CAMPELLO. -NOTIFICACIÓN DE INICIACIÓN DE EXPEDIENTES -NOTIFICACIÓN DENUNCIAS DE TRÁFICO 110 SANCIONADORES 24 -NOTIFICACIÓN RESOLUCIONES 40 AYUNTAMIENTO CAÑADA. -NOTIFICACIÓN RESOLUCIONES 41 -APROBACIÓN DEFINITIVA PROYECTO REPARCELACIÓN FORZOSA -NOTIFICACIÓN RESOLUCIONES 42 UNIDAD EJECUCIÓN ÚNICA PLAN REFORMA INTERIOR ÁMBITO -NOTIFICACIÓN INICIACIÓN EXPEDIENTES SANCIONADORES 45 SUELO URBANO INDUSTRIAL SECTOR PDI-1 111 SERVICIO DE COSTAS ALICANTE. AYUNTAMIENTO COCENTAINA. -NOTIFICACIÓN RESOLUCIÓN EXPEDIENTE SANCIONADOR 46 -CORRECCIÓN DE ERRORES EDICTO PUBLICADO -

Women, Business and the Law 2020 World Bank Group
WOMEN, BUSINESS AND THE LAW 2020 AND THE LAW BUSINESS WOMEN, WOMEN, BUSINESS AND THE LAW 2020 WORLD BANK GROUP WORLD WOMEN, BUSINESS AND THE LAW 2020 © 2020 International Bank for Reconstruction and Development / The World Bank 1818 H Street NW, Washington, DC 20433 Telephone: 202-473-1000; Internet: www.worldbank.org Some rights reserved 1 2 3 4 23 22 21 20 This work is a product of the staff of The World Bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the govern- ments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Nothing herein shall constitute or be considered to be a limitation upon or waiver of the privileges and immunities of The World Bank, all of which are specifically reserved. Rights and Permissions This work is available under the Creative Commons Attribution 3.0 IGO license (CC BY 3.0 IGO) http://creativecommons.org/ licenses/by/3.0/igo. Under the Creative Commons Attribution license, you are free to copy, distribute, transmit, and adapt this work, including for commercial purposes, under the following conditions: Attribution—Please cite the work as follows: World Bank. 2020. Women, Business and the Law 2020. -

Men Final Entries
Final Entries - Athletes List by event European Athletics Indoor Championships 2021, Torun/POL Tot. Number of countries Tot. Number of athletes Tot. Number of Men Tot. Number of Women 47 733 405 328 FINAL ENTRIES - Men 60m Senior Men Num. of countries: 33 Num. of athletes: 71 Member Federation Surname First Name DoB PB SB ARM Donigian Alexander 20/10/1993 6.64i 6.79i ART Keletela Dorian Celeste 06/02/1999 6.79i 6.85i AUT Fuchs Markus 14/11/1995 6.62i 6.69i BEL Kuba Di-Vita Gaylord 17/11/1995 6.73i 6.75i BEL Vleminckx Kobe 31/05/1998 6.65i 6.65i BLR Bliznets Dzianis 12/03/1995 6.75i 6.75i BLR Bohdan Maksim 19/03/1997 6.77i 6.77i BLR Zabalotny Yury 24/02/1997 6.72i 6.72i BUL Dimitrov Denis 10/02/1994 6.65i 6.73i BUL Jivkov Vesselin 26/01/2001 6.76i 6.80i CZE Hampl Štěpán 10/11/1999 6.70i 6.70i CZE Stromšík Zdeněk 25/11/1994 6.60i 6.68i CZE Veleba Jan 06/12/1986 6.65i 6.65i DEN Hansen Simon 30/06/1998 6.75i 6.75i DEN Kjær Emil Mader 20/12/1999 6.77i 6.77i DEN Musah Kojo 15/04/1996 6.61i 6.61i ESP López Sergio 05/07/1999 6.67i 6.74i ESP Rodríguez Daniel 26/01/1995 6.67i 6.67i ESP Sanchez Ricardo 10/08/1999 6.75i 6.75i EST Nazarov Karl Erik 17/03/1999 6.63i 6.63i FIN Illukka Riku 21/09/1999 6.73i 6.73i FIN Purola Samuel 19/05/2000 6.67i 6.67i FIN Samuelsson Samuli 23/06/1995 6.66i 6.66i FRA Fall Mouhamadou 25/02/1992 6.62i 6.62i FRA Golitin Amaury 28/01/1997 6.62i 6.62i GBR Aikines-Aryeetey Harry 29/08/1988 6.55i 6.67i GBR Bromby Oliver 30/03/1998 6.63i 6.65i GBR Robertson Andrew 17/12/1990 6.54i 6.61i GER Corucle Philipp 18/07/1997 6.62i -

Peer-Reviewed Articles______Works Are Reported in Chronological Order
Prof. Giulia Fulvia Mancini – List of Publications Peer-reviewed Articles_____________________________________________________________ Works are reported in chronological order. O. Cannelli, N. Colonna, M. Puppin, T. Rossi, D. Kinschel, L. Leroy, J. Löffler, A. M. March, G. Doumy, A. Al Haddad, M.-F. Tu, Y. Kumagai, D. Walko, G. Smolentsev, F. Krieg, S. C. Boehme, M. V. Kovalenko, M. Chergui, G. F. Mancini, "Quantifying Photoinduced Polaronic Distortions in Inorganic Lead Halide Perovskites Nanocrystals", J.Am.Chem. Soc. (2021) - Accepted. J. R. Rouxel, D. Fainozzi, R. Mankowsky, B. Rösner, G. Seniutinas, R. Mincigrucci, S. Catalini, L. Foglia, R. Cucini, F. Döring, A. Kubec, F. Koch, F. Bencivenga, A. Al Haddad, A. Gessini, A. A. Maznev, C. Cirelli, S. Gerber, B. Pedrini, G. F. Mancini, E. Razzoli, M. Burian, H. Ueda, G. Pamfilidis, E. Ferrari, Y. Deng, A. Mozzanica, P. J. M. Johnson, D. Ozerov, M. G. Izzo, C. Bottari, C. Arrell, E. J. Divall, Serhane Zerdane, M. Sander, G. Knopp, P. Beaud, H. T. Lemke, C. J. Milne, C. David, R. Torre, M. Chergui, K. A. Nelson, C. Masciovecchio, U. Staub, L. Patthey, C. Svetina, Hard X-ray transient grating spectroscopy on bismuth germanate, Nat. Photonics (2021). https://doi.org/10.1038/s41566-021-00797-9 C. Bacellar, D. Kinschel, O. Cannelli, B. Sorokin, T. Katayama, G. F. Mancini, J. R. Rouxel, Y. Obara, J. Nishitani, H. Ito, T. Ito, N. Kurahashi, C. Higashimura, S. Kudo, C. Cirelli, G. Knopp, K. Nass, P. J. M. Johnson, A. Wach, J. Szlachetko, F. A. Lima, C. J. Milne, M. Yabashi, T. Suzuki, K. Misawac, M. Chergui, "Femtosecond X-ray spectroscopy of haem proteins", Faraday Discuss., Advance Article (2021). -

Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -

See Who Attended
Company Name First Name Last Name Job Title Country 24Sea Gert De Sitter Owner Belgium 2EN S.A. George Droukas Data analyst Greece 2EN S.A. Yannis Panourgias Managing Director Greece 3E Geert Palmers CEO Belgium 3E Baris Adiloglu Technical Manager Belgium 3E David Schillebeeckx Wind Analyst Belgium 3E Grégoire Leroy Product Manager Wind Resource Modelling Belgium 3E Rogelio Avendaño Reyes Regional Manager Belgium 3E Luc Dewilde Senior Business Developer Belgium 3E Luis Ferreira Wind Consultant Belgium 3E Grégory Ignace Senior Wind Consultant Belgium 3E Romain Willaime Sales Manager Belgium 3E Santiago Estrada Sales Team Manager Belgium 3E Thomas De Vylder Marketing & Communication Manager Belgium 4C Offshore Ltd. Tom Russell Press Coordinator United Kingdom 4C Offshore Ltd. Lauren Anderson United Kingdom 4Cast GmbH & Co. KG Horst Bidiak Senior Product Manager Germany 4Subsea Berit Scharff VP Offshore Wind Norway 8.2 Consulting AG Bruno Allain Président / CEO Germany 8.2 Consulting AG Antoine Ancelin Commercial employee Germany 8.2 Monitoring GmbH Bernd Hoering Managing Director Germany A Word About Wind Zoe Wicker Client Services Manager United Kingdom A Word About Wind Richard Heap Editor-in-Chief United Kingdom AAGES Antonio Esteban Garmendia Director - Business Development Spain ABB Sofia Sauvageot Global Account Executive France ABB Jesús Illana Account Manager Spain ABB Miguel Angel Sanchis Ferri Senior Product Manager Spain ABB Antoni Carrera Group Account Manager Spain ABB Luis andres Arismendi Gomez Segment Marketing Manager Spain -

NOSTALGIA, EMOTIONALITY, and ETHNO-REGIONALISM in PONTIC PARAKATHI SINGING by IOANNIS TSEKOURAS DISSERTATION Submitted in Parti
NOSTALGIA, EMOTIONALITY, AND ETHNO-REGIONALISM IN PONTIC PARAKATHI SINGING BY IOANNIS TSEKOURAS DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Musicology in the Graduate College of the University of Illinois at Urbana-Champaign, 2016 Urbana, Illinois Doctoral Committee: Associate Professor Donna A. Buchanan, Chair Professor Emeritus Thomas Turino Professor Gabriel Solis Professor Maria Todorova ABSTRACT This dissertation explores the multilayered connections between music, emotionality, social and cultural belonging, collective memory, and identity discourse. The ethnographic case study for the examination of all these relations and aspects is the Pontic muhabeti or parakathi. Parakathi refers to a practice of socialization and music making that is designated insider Pontic Greek. It concerns primarily Pontic Greeks or Pontians, the descendants of the 1922 refugees from Black Sea Turkey (Gr. Pontos), and their identity discourse of ethno-regionalism. Parakathi references nightlong sessions of friendly socialization, social drinking, and dialogical participatory singing that take place informally in coffee houses, taverns, and households. Parakathi performances are reputed for their strong Pontic aesthetics, traditional character, rich and aesthetically refined repertoire, and intense emotionality. Singing in parakathi performances emerges spontaneously from verbal socialization and emotional saturation. Singing is described as a confessional expression of deeply personal feelings -

Village Social Organisation and Peasant Action: Right-Bank Ukraine During the Revolution 1917-1923
VILLAGE SOCIAL ORGANISATION AND PEASANT ACTION: RIGHT-BANK UKRAINE DURING THE REVOLUTION I9I7-I923 GRAHAM TAN PhD SCHOOL OF SLAVONIC AND EAST EUROPEAN STUDIES UNIVERSITY OF LONDON » UNIVERSITY ) " F J . LONOOf,' ' / ProQuest Number: U642459 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U642459 Published by ProQuest LLC(2015). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT 2 VILLAGE SOCIAL ORGANISATION AND PEASANT ACTION: RIGHT’-BANK UKRAINE DURING THE REVOLUTION 1917-1923 The thesis studies the role of peasant village institutions in the revolution in Right-Bank Ukraine during 1917-1923. The two schools of study which have so far dominated discussion of the subject, the Soviet and the Ukrainian National, have failed to produce a balanced history of events or follow the recent progress made in studies of the Russian peasantry. The work studies events from a village-level perspective and is based on records from peasant meetings and local government institutions, gathered from recently declassified fonds in Ukrainian and Russian archives. The thesis begins by considering the roots of the region’s economic and political diversity and their effect on peasant society before 1917. -

Diplomatic List – Fall 2018
United States Department of State Diplomatic List Fall 2018 Preface This publication contains the names of the members of the diplomatic staffs of all bilateral missions and delegations (herein after “missions”) and their spouses. Members of the diplomatic staff are the members of the staff of the mission having diplomatic rank. These persons, with the exception of those identified by asterisks, enjoy full immunity under provisions of the Vienna Convention on Diplomatic Relations. Pertinent provisions of the Convention include the following: Article 29 The person of a diplomatic agent shall be inviolable. He shall not be liable to any form of arrest or detention. The receiving State shall treat him with due respect and shall take all appropriate steps to prevent any attack on his person, freedom, or dignity. Article 31 A diplomatic agent shall enjoy immunity from the criminal jurisdiction of the receiving State. He shall also enjoy immunity from its civil and administrative jurisdiction, except in the case of: (a) a real action relating to private immovable property situated in the territory of the receiving State, unless he holds it on behalf of the sending State for the purposes of the mission; (b) an action relating to succession in which the diplomatic agent is involved as an executor, administrator, heir or legatee as a private person and not on behalf of the sending State; (c) an action relating to any professional or commercial activity exercised by the diplomatic agent in the receiving State outside of his official functions. -- A diplomatic agent’s family members are entitled to the same immunities unless they are United States Nationals. -

Monocot Fossils Suitable for Molecular Dating Analyses
bs_bs_banner Botanical Journal of the Linnean Society, 2015, 178, 346–374. With 1 figure INVITED REVIEW Monocot fossils suitable for molecular dating analyses WILLIAM J. D. ILES1,2*, SELENA Y. SMITH3, MARIA A. GANDOLFO4 and SEAN W. GRAHAM1 1Department of Botany, University of British Columbia, 3529-6270 University Blvd, Vancouver, BC, Canada V6T 1Z4 2University and Jepson Herbaria, University of California, Berkeley, 3101 Valley Life Sciences Bldg, Berkeley, CA 94720-3070, USA 3Department of Earth & Environmental Sciences and Museum of Paleontology, University of Michigan, 2534 CC Little Bldg, 1100 North University Ave., Ann Arbor, MI 48109-1005, USA 4LH Bailey Hortorium, Plant Biology Section, School of Integrative Plant Science, Cornell University, 410 Mann Library Bldg, Ithaca, NY 14853, USA Received 6 June 2014; revised 3 October 2014; accepted for publication 7 October 2014 Recent re-examinations and new fossil findings have added significantly to the data available for evaluating the evolutionary history of the monocotyledons. Integrating data from the monocot fossil record with molecular dating techniques has the potential to help us to understand better the timing of important evolutionary events and patterns of diversification and extinction in this major and ancient clade of flowering plants. In general, the oldest well-placed fossils are used to constrain the age of nodes in molecular dating analyses. However, substantial error can be introduced if calibration fossils are not carefully evaluated and selected. Here we propose a set of 34 fossils representing 19 families and eight orders for calibrating the ages of major monocot clades. We selected these fossils because they can be placed in particular clades with confidence and they come from well-dated stratigraphic sequences.