3. Some One-Dimensional Potentials This “Tillegg” Is a Supplement to Sections 3.1, 3.3 and 3.5 in Hemmer’S Book
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dirac -Function Potential in Quasiposition Representation of a Minimal-Length Scenario
Eur. Phys. J. C (2018) 78:179 https://doi.org/10.1140/epjc/s10052-018-5659-6 Regular Article - Theoretical Physics Dirac δ-function potential in quasiposition representation of a minimal-length scenario M. F. Gusson, A. Oakes O. Gonçalves, R. O. Francisco, R. G. Furtado, J. C. Fabrisa, J. A. Nogueirab Departamento de Física, Universidade Federal do Espírito Santo, Vitória, ES 29075-910, Brazil Received: 26 April 2017 / Accepted: 22 February 2018 / Published online: 3 March 2018 © The Author(s) 2018. This article is an open access publication Abstract A minimal-length scenario can be considered as Although the first proposals for the existence of a mini- an effective description of quantum gravity effects. In quan- mal length were done by the beginning of 1930s [1–3], they tum mechanics the introduction of a minimal length can were not connected with quantum gravity, but instead with be accomplished through a generalization of Heisenberg’s a cut-off in nature that would remedy cumbersome diver- uncertainty principle. In this scenario, state eigenvectors of gences arising from quantization of systems with an infinite the position operator are no longer physical states and the number of degrees of freedom. The relevant role that grav- representation in momentum space or a representation in a ity plays in trying to probe a smaller and smaller region of quasiposition space must be used. In this work, we solve the the space-time was recognized by Bronstein [4] already in Schroedinger equation with a Dirac δ-function potential in 1936; however, his work did not attract a lot of attention. -

The Scattering and Shrinking of a Gaussian Wave Packet by Delta Function Potentials Fei
The Scattering and Shrinking of a Gaussian Wave Packet by Delta Function Potentials by Fei Sun Submitted to the Department of Physics ARCHIVF in partial fulfillment of the requirements for the degree of Bachelor of Science r U at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2012 @ Massachusetts Institute of Technology 2012. All rights reserved. A u th or ........................ ..................... Department of Physics May 9, 2012 C ertified by .................. ...................... ................ Professor Edmund Bertschinger Thesis Supervisor, Department of Physics Thesis Supervisor Accepted by.................. / V Professor Nergis Mavalvala Senior Thesis Coordinator, Department of Physics The Scattering and Shrinking of a Gaussian Wave Packet by Delta Function Potentials by Fei Sun Submitted to the Department of Physics on May 9, 2012, in partial fulfillment of the requirements for the degree of Bachelor of Science Abstract In this thesis, we wish to test the hypothesis that scattering by a random potential causes localization of wave functions, and that this localization is governed by the Born postulate of quantum mechanics. We begin with a simple model system: a one-dimensional Gaussian wave packet incident from the left onto a delta function potential with a single scattering center. Then we proceed to study the more compli- cated models with double and triple scattering centers. Chapter 1 briefly describes the motivations behind this thesis and the phenomenon related to this research. Chapter 2 to Chapter 4 give the detailed calculations involved in the single, double and triple scattering cases; for each case, we work out the exact expressions of wave functions, write computer programs to numerically calculate the behavior of the wave packets, and use graphs to illustrate the results of the calculations. -

Scattering from the Delta Potential
Phys 341 Quantum Mechanics Day 10 Wed. 9/24 2.5 Scattering from the Delta Potential (Q7.1, Q11) Computational: Time-Dependent Discrete Schro Daily 4.W 4 Science Poster Session: Hedco7pm~9pm Fri., 9/26 2.6 The Finite Square Well (Q 11.1-.4) beginning Daily 4.F Mon. 9/29 2.6 The Finite Square Well (Q 11.1-.4) continuing Daily 5.M Tues 9/30 Weekly 5 5 Wed. 10/1 Review Ch 1 & 2 Daily 5.W Fri. 10/3 Exam 1 (Ch 1 & 2) Equipment Load our full Python package on computer Comp 5: discrete Time-Dependent Schro Griffith’s text Moore’s text Printout of roster with what pictures I have Check dailies Announcements: Daily 4.W Wednesday 9/24 Griffiths 2.5 Scattering from the Delta Potential (Q7.1, Q11) 1. Conceptual: State the rules from Q11.4 in terms of mathematical equations. Can you match the rules to equations in Griffiths? If you can, give equation numbers. 7. Starting Weekly, Computational: Follow the instruction in the handout “Discrete Time- Dependent Schrodinger” to simulate a Gaussian packet’s interacting with a delta-well. 2.5 The Delta-Function Potential 2.5.1 Bound States and Scattering States 1. Conceptual: Compare Griffith’s definition of a bound state with Q7.1. 2. Conceptual: Compare Griffith’s definition of tunneling with Q11.3. 3. Conceptual: Possible energy levels are quantized for what kind of states (bound, and/or unbound)? Why / why not? Griffiths seems to bring up scattering states out of nowhere. By scattering does he just mean transmission and reflection?" Spencer 2.5.2 The Delta-Function Recall from a few days ago that we’d encountered sink k a 0 for k ko 2 o k ko 2a for k ko 1 Phys 341 Quantum Mechanics Day 10 When we were dealing with the free particle, and we were planning on eventually sending the width of our finite well to infinity to arrive at the solution for the infinite well. -

Quantum Mechanics
Quantum Mechanics Richard Fitzpatrick Professor of Physics The University of Texas at Austin Contents 1 Introduction 5 1.1 Intendedaudience................................ 5 1.2 MajorSources .................................. 5 1.3 AimofCourse .................................. 6 1.4 OutlineofCourse ................................ 6 2 Probability Theory 7 2.1 Introduction ................................... 7 2.2 WhatisProbability?.............................. 7 2.3 CombiningProbabilities. ... 7 2.4 Mean,Variance,andStandardDeviation . ..... 9 2.5 ContinuousProbabilityDistributions. ........ 11 3 Wave-Particle Duality 13 3.1 Introduction ................................... 13 3.2 Wavefunctions.................................. 13 3.3 PlaneWaves ................................... 14 3.4 RepresentationofWavesviaComplexFunctions . ....... 15 3.5 ClassicalLightWaves ............................. 18 3.6 PhotoelectricEffect ............................. 19 3.7 QuantumTheoryofLight. .. .. .. .. .. .. .. .. .. .. .. .. .. 21 3.8 ClassicalInterferenceofLightWaves . ...... 21 3.9 QuantumInterferenceofLight . 22 3.10 ClassicalParticles . .. .. .. .. .. .. .. .. .. .. .. .. .. .. 25 3.11 QuantumParticles............................... 25 3.12 WavePackets .................................. 26 2 QUANTUM MECHANICS 3.13 EvolutionofWavePackets . 29 3.14 Heisenberg’sUncertaintyPrinciple . ........ 32 3.15 Schr¨odinger’sEquation . 35 3.16 CollapseoftheWaveFunction . 36 4 Fundamentals of Quantum Mechanics 39 4.1 Introduction .................................. -

Electric Polarizability in the Three Dimensional Problem and the Solution of an Inhomogeneous Differential Equation
Electric polarizability in the three dimensional problem and the solution of an inhomogeneous differential equation M. A. Maizea) and J. J. Smetankab) Department of Physics, Saint Vincent College, Latrobe, PA 15650 USA In previous publications, we illustrated the effectiveness of the method of the inhomogeneous differential equation in calculating the electric polarizability in the one-dimensional problem. In this paper, we extend our effort to apply the method to the three-dimensional problem. We calculate the energy shift of a quantum level using second-order perturbation theory. The energy shift is then used to calculate the electric polarizability due to the interaction between a static electric field and a charged particle moving under the influence of a spherical delta potential. No explicit use of the continuum states is necessary to derive our results. I. INTRODUCTION In previous work1-3, we employed the simple and elegant method of the inhomogeneous differential equation4 to calculate the energy shift of a quantum level in second-order perturbation theory. The energy shift was a result of an interaction between an applied static electric field and a charged particle moving under the influence of a one-dimensional bound 1 potential. The electric polarizability, , was obtained using the basic relationship ∆퐸 = 휖2 0 2 with ∆퐸0 being the energy shift and is the magnitude of the applied electric field. The method of the inhomogeneous differential equation devised by Dalgarno and Lewis4 and discussed by Schwartz5 can be used in a large variety of problems as a clever replacement for conventional perturbation methods. As we learn in our introductory courses in quantum mechanics, calculating the energy shift in second-order perturbation using conventional methods involves a sum (which can be infinite) or an integral that contains all possible states allowed by the transition. -

Revisiting Double Dirac Delta Potential
Revisiting double Dirac delta potential Zafar Ahmed1, Sachin Kumar2, Mayank Sharma3, Vibhu Sharma3;4 1Nuclear Physics Division, Bhabha Atomic Research Centre, Mumbai 400085, India 2Theoretical Physics Division, Bhabha Atomic Research Centre, Mumbai 400085, India 3;4Amity Institute of Applied Sciences, Amity University, Noida, UP, 201313, India∗ (Dated: June 14, 2016) Abstract We study a general double Dirac delta potential to show that this is the simplest yet versatile solvable potential to introduce double wells, avoided crossings, resonances and perfect transmission (T = 1). Perfect transmission energies turn out to be the critical property of symmetric and anti- symmetric cases wherein these discrete energies are found to correspond to the eigenvalues of Dirac delta potential placed symmetrically between two rigid walls. For well(s) or barrier(s), perfect transmission [or zero reflectivity, R(E)] at energy E = 0 is non-intuitive. However, earlier this has been found and called \threshold anomaly". Here we show that it is a critical phenomenon and we can have 0 ≤ R(0) < 1 when the parameters of the double delta potential satisfy an interesting condition. We also invoke zero-energy and zero curvature eigenstate ( (x) = Ax + B) of delta well between two symmetric rigid walls for R(0) = 0. We resolve that the resonant energies and the perfect transmission energies are different and they arise differently. arXiv:1603.07726v4 [quant-ph] 13 Jun 2016 ∗Electronic address: 1:[email protected], 2: [email protected], 3: [email protected], 4:[email protected] 1 I. INTRODUCTION The general one-dimensional Double Dirac Delta Potential (DDDP) is written as [see Fig. -

Paradoxical Quantum Scattering Off a Time Dependent Potential?
Paradoxical Quantum Scattering off a Time Dependent Potential? Ori Reinhardt and Moshe Schwartz Department of Physics, Raymond and Beverly Sackler Faculty Exact Sciences, Tel Aviv University, Tel Aviv 69978, Israel We consider the quantum scattering off a time dependent barrier in one dimension. Our initial state is a right going eigenstate of the Hamiltonian at time t=0. It consists of a plane wave incoming from the left, a reflected plane wave on the left of the barrier and a transmitted wave on its right. We find that at later times, the evolving wave function has a finite overlap with left going eigenstates of the Hamiltonian at time t=0. For simplicity we present an exact result for a time dependent delta function potential. Then we show that our result is not an artifact of that specific choice of the potential. This surprising result does not agree with our interpretation of the eigenstates of the Hamiltonian at time t=0. A numerical study of evolving wave packets, does not find any corresponding real effect. Namely, we do not see on the right hand side of the barrier any evidence for a left going packet. Our conclusion is thus that the intriguing result mentioned above is intriguing only due to the semantics of the interpretation. PACS numbers: 03.65.-w, 03.65.Nk, 03.65.Xp, 66.35.+a The quantum problem of an incoming plain wave The eigenstates of the Hamiltonian (Eq. 1) can be encountering a static potential barrier in one dimension is classified according to the absolute value of incoming an old canonical text book example in which the reflection momentum and to whether they are right or left going. -

Casimir Effect for Impurity in Periodic Background in One Dimension
Journal of Physics A: Mathematical and Theoretical J. Phys. A: Math. Theor. 53 (2020) 325401 (24pp) https://doi.org/10.1088/1751-8121/ab9463 Casimir effect for impurity in periodic background in one dimension M Bordag Institute for Theoretical Physics, Universität Leipzig Brüderstraße 16 04103 Leipzig, Germany E-mail: [email protected] Received 2 April 2020, revised 7 May 2020 Accepted for publication 19 May 2020 Published 27 July 2020 Abstract We consider a Bose gas in a one-dimensional periodic background formed by generalized delta function potentials with one and two impurities. We inves- tigate the scattering off these impurities and their bound state levels. Besides expected features, we observe a kind of long-range correlation between the bound state levels of two impurities. Further, we define and calculate the vac- uum energy of the impurity. It causes a force acting on the impurity relative to the background. We define the vacuum energy as a mode sum. In order toget a discrete spectrum we start from a finite lattice and use Chebychev polynomi- als to get a general expression. These allow also for quite easy investigation of impurities in finite lattices. Keywords: quantum vacuum, Casimir effect (theory), periodic potential (Some figures may appear in colour only in the online journal) 1. Introduction One-dimensional systems provide a large number of examples to study quantum effects. These allow frequently for quite explicit and instructive formulas, but at the same time there are many one-dimensional and quasi-one-dimensional systems in physics which are frequently also of practical interest like nanowires and carbon nanotubes. -
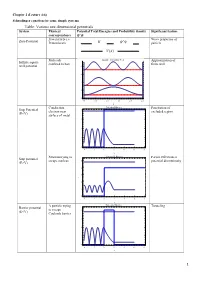
Table: Various One Dimensional Potentials System Physical Potential Total Energies and Probability Density Significant Feature Correspondence Free Particle I.E
Chapter 2 (Lecture 4-6) Schrodinger equation for some simple systems Table: Various one dimensional potentials System Physical Potential Total Energies and Probability density Significant feature correspondence Free particle i.e. Wave properties of Zero Potential Proton beam particle Molecule Infinite Potential Well Approximation of Infinite square confined to box finite well well potential 8 6 4 2 0 0.0 0.5 1.0 1.5 2.0 2.5 x Conduction Potential Barrier Penetration of Step Potential 4 electron near excluded region (E<V) surface of metal 3 2 1 0 4 2 0 2 4 6 8 x Neutron trying to Potential Barrier Partial reflection at Step potential escape nucleus 5 potential discontinuity (E>V) 4 3 2 1 0 4 2 0 2 4 6 8 x Α particle trying Potential Barrier Tunneling Barrier potential to escape 4 (E<V) Coulomb barrier 3 2 1 0 4 2 0 2 4 6 8 x 1 Electron Potential Barrier No reflection at Barrier potential 5 scattering from certain energies (E>V) negatively 4 ionized atom 3 2 1 0 4 2 0 2 4 6 8 x Neutron bound in Finite Potential Well Energy quantization Finite square well the nucleus potential 4 3 2 1 0 2 0 2 4 6 x Aromatic Degenerate quantum Particle in a ring compounds states contains atomic rings. Model the Quantization of energy Particle in a nucleus with a and degeneracy of spherical well potential which is or states zero inside the V=0 nuclear radius and infinite outside that radius. -

Tunneling in Fractional Quantum Mechanics
Tunneling in Fractional Quantum Mechanics E. Capelas de Oliveira1 and Jayme Vaz Jr.2 Departamento de Matem´atica Aplicada - IMECC Universidade Estadual de Campinas 13083-859 Campinas, SP, Brazil Abstract We study the tunneling through delta and double delta po- tentials in fractional quantum mechanics. After solving the fractional Schr¨odinger equation for these potentials, we cal- culate the corresponding reflection and transmission coeffi- cients. These coefficients have a very interesting behaviour. In particular, we can have zero energy tunneling when the order of the Riesz fractional derivative is different from 2. For both potentials, the zero energy limit of the transmis- sion coefficient is given by = cos2 (π/α), where α is the T0 order of the derivative (1 < α 2). ≤ 1. Introduction In recent years the study of fractional integrodifferential equations applied to physics and other areas has grown. Some examples are [1, 2, 3], among many others. More recently, the fractional generalized Langevin equation is proposed to discuss the anoma- lous diffusive behavior of a harmonic oscillator driven by a two-parameter Mittag-Leffler noise [4]. Fractional Quantum Mechanics (FQM) is the theory of quantum mechanics based on the fractional Schr¨odinger equation (FSE). In this paper we consider the FSE as introduced by Laskin in [5, 6]. It was obtained in the context of the path integral approach to quantum mechanics. In this approach, path integrals are defined over L´evy flight paths, which is a natural generalization of the Brownian motion [7]. arXiv:1011.1948v2 [math-ph] 15 Mar 2011 There are some papers in the literature studying solutions of FSE. -

Some Exact Results for the Schršdinger Wave Equation with a Time Dependent Potential Joel Campbell NASA Langley Research Center
Some Exact Results for the Schršdinger Wave Equation with a Time Dependent Potential Joel Campbell NASA Langley Research Center, MS 488 Hampton, VA 23681 [email protected] Abstract The time dependent Schršdinger equation with a time dependent delta function potential is solved exactly for many special cases. In all other cases the problem can be reduced to an integral equation of the Volterra type. It is shown that by knowing the wave function at the origin, one may derive the wave function everywhere. Thus, the problem is reduced from a PDE in two variables to an integral equation in one. These results are used to compare adiabatic versus sudden changes in the potential. It is shown that adiabatic changes in the p otential lead to conservation of the normalization of the probability density. 02.30Em, 02.60-x, 03.65-w, 61.50-f Introduction Very few cases of the time dependent Schršdinger wave equation with a time dependent potential can be solved exactly. The cases that are known include the time dependent harmonic oscillator,[ 1-3] an example of an infinite potential well with a moving boundary,[4-6] and various other special cases. There remain, however, a large number of time dependent problems that, at least in principal, can be solved exactly. One case that has been looked at over the years is the delta function potential. The author first investigated this problem in the mid 1980s [7] as an unpublished work. This was later included as part of a PhD thesis dissertation [8] but not published in a journal. -

The Attractive Nonlinear Delta-Function Potential
The Attractive Nonlinear Delta-Function Potential M. I. Molina and C. A. Bustamante Facultad de Ciencias, Departamento de F´ısica, Universidad de Chile Casilla 653, Las Palmeras 3425, Santiago, Chile. arXiv:physics/0102053v1 [physics.ed-ph] 16 Feb 2001 1 Abstract We solve the continuous one-dimensional Schr¨odinger equation for the case of an inverted nonlinear delta–function potential located at the origin, ob- taining the bound state in closed form as a function of the nonlinear expo- nent. The bound state probability profile decays exponentially away from the origin, with a profile width that increases monotonically with the non- linear exponent, becoming an almost completely extended state when this approaches two. At an exponent value of two, the bound state suffers a discontinuous change to a delta–like profile. Further increase of the expo- nent increases again the width of the probability profile, although the bound state is proven to be stable only for exponents below two. The transmission of plane waves across the nonlinear delta potential increases monotonically with the nonlinearity exponent and is insensitive to the sign of its opacity. 2 The delta-function potential δ(x x ) has become a familiar sight in the − 0 landscape of most elementary courses on quantum mechanics, where it serves to illustrate the basic techniques in simple form. As a physical model, it has been used to represent a localized potential whose energy scale is greater than any other in the problem at hand and whose spatial extension is smaller than other relevant length scales of the problem. Arrays of delta-function potentials have been used to illustrate Bloch’s theorem in solid state physics and also in optics, where in the scalar approximation, wave propagation in a periodic medium resembles the dynamics of an electron in a crystal lattice.