Structural and Kinetic Study of Rhodium Complexes of N-Aryl-N-Nitrosohydroxylamines and Related Complexes
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Nigam Prasad Rath Research Professor
Nigam Prasad Rath Research Professor Department of Chemistry and Biochemistry University of Missouri - St. Louis One University Boulevard St. Louis, MO 63121. E-mail: [email protected] Phone: 314-516-5333 FAX: 314-516-5342 Education : B. Sc.(Honors) : 1st Class Honors in Chemistry with Distinction, Berhampur University, Berhampur, India, 1977. M. Sc. (Chemistry): 1st Class, Berhampur University, Berhampur, India, 1979. Ph. D. (Chemistry): Oklahoma State University, Stillwater, OK, USA, 1985. Professional Experience: Research Professor , Department of Chemistry and Biochemistry, University of Missouri, St. Louis, MO, 2004 to present. Research Associate Professor , Department of Chemistry, University of Missouri, St. Louis, MO, 1997 to 2004. Research Assistant Professor , Department of Chemistry, University of Missouri, St. Louis, MO, 1989 to 1996. Assistant Faculty Fellow , Department of Chemistry, University of Notre Dame, Notre Dame, IN 1987 to 1989. Post Doctoral Research Associate , Department of Chemistry, University of Notre Dame, Notre Dame, IN 1986-87. Graduate Assistant , Department of Chemistry, Oklahoma State University, Stillwater, OK 1982 to 1985. Junior Research Fellow (CSIR) , Department of Chemistry, Indian Institute of Technology, Kharagpur, India, 1981-82. Junior Research Fellow , Department of Chemistry, Indian Institute of Technology, Kanpur, India, 1979 to 1981. 2 Professional Positions: Visiting Scientist, Monsanto Corporate Research, Chesterfield, MO, 1992 to 1994. Scientific Consultant, Regional Research Laboratory, Trivandrum, India, 1992 to present. Assistant Professor, Evening College, University of Missouri, St. Louis, 1992 to 2000. Research Mentor, Engelmann Mathematics and Science Institute, University of Missouri, St. Louis, 1990 to 1998. Research Mentor, NSF STARS Program, University of Missouri, St. Louis, 1999 to present. Honors and Awards: National Merit Scholarship, India, 1977-79. -

United States Patent Office Patented Apr
3,803,252 United States Patent Office Patented Apr. 9, 1974 1. 2 3,803,252 in which R is as hereinbefore defined and Y represents PROCESS FOR THE PREPARATION OF CAROTENOD COMPOUNDS a hydrogen atom or a retinyl radical. This formula may Pierre Chabardes, Lyon, and Marc Julia, Paris, France, represent sterically pure products, or mixtures of differ assignors to Rhone-Poulenc S.A., Paris, France ent isomers. 5 The unsubstituted sulphones used as starting mate No Drawing. Filed May 17, 1972, Ser. No. 254,103 rials have the Formula I in which Y represents a hy Claims priority, applicitFrance, May 19, 1971, drogen atom; they are called herein retinyl-Sulphones. Int, C. C07c9 13/00 They can be prepared by known methods for the prep U.S. C. 260-666 C 9 Claims aration of sulphones. A particularly advantageous method O consists of reacting an alkali metal sulphinate of the formula RSOM, in which R represents alkyl, aryl, alkyl ABSTRACT OF THE DISCLOSURE aryl or aralkyl, preferably phenyl or tolyl, and M rep Caroteine compounds e.g. g-caroteine, are prepared by resents an alkali metal, with retinol or a retinol ester reacting a sulphone Ret-SO-R, in which Ret repre of an inorganic or organic acid, such as retinyl chloride sents retinyl or substituted retinyl and R represents a 15 or bromide, or retinyl formate or acetate. They can also hydrocarbon radical, in the presence of an alkaline be prepared by reacting a sulphinic acid RSOH with reagent with an ester Ret-X, in which X represents an retinol, it being possible to form this acid in situ, if re acid residue, and then desulphonating the product, for quired, from a metal sulphinate in an acid medium. -

United States Patent (19) 11 4,311,652 Abramson Et Al
United States Patent (19) 11 4,311,652 Abramson et al. 45 Jan. 19, 1982 54 ARBUZOV REACTIONS EMPLOYING AN 56) References Cited ALEPHATIC SOLVENT U.S. PATENT DOCUMENTS 3,483,279 12/1969 Davis et al. ......................... 260/969 75 Inventors: Alan Abramson, White Plains; 3,699,193 10/1972 Melton ..... ... 260/969 Edward D. Weil, 3,776,984 12/1973 Ratts ................................... 260/969 Hastings-on-Hudson, both of N.Y. Primary Examinter-Anton H. Sutto Attorney, Agent, or Firm-Vivienne T. White 73 Assignee: Stauffer Chemical Company, 57 ABSTRACT Westport, Conn. An improved process for phosphite rearrangement wherein the phosphite is rearranged to the phospho nate. The improvement comprises conducting the phos 21 Appl. No.: 154,170 phite rearrangement reaction in an aliphatic solvent which is miscible with the reactants at reaction temper atures, and immiscible with the product at lower tem 22 Filed: May 28, 1980 peratures. Increased yields of the phosphonate product are obtained without the need for additional distillation 51 int. Cl. ................................................ C07F 9/40 for solvent separation. 52 U.S. C. ................ ... 260/969; 260/990 58 Field of Search ................................ 260/969, 990 12 Claims, No Drawings 4,311,652 3 4. The advantage of conducting the rearrangement in a process for rearranging tris(2-chloroethyl) phosphite. solvent were found to be a more easily controlled exo The invention is directed to phosphite rearrangements, therm due to the heating and reflux of the solvent which wherein the novel process comprises conducting the removes the heat of reaction, and inhibiting the degra rearrangement process in an essentially aliphatic Solvent dation of the phosphonate product produced. -

Synthetic, Sterochemical, and Electronic Studies of Organophosphorous Ligands and Their Transition Metal Complexes James Timothy Spencer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1984 Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes James Timothy Spencer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Spencer, James Timothy, "Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes " (1984). Retrospective Theses and Dissertations. 7727. https://lib.dr.iastate.edu/rtd/7727 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Synthesis of 4-Phosphono Β-Lactams and Related Azaheterocyclic Phosphonates
SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES IR . KRISTOF MOONEN To Elza Vercauteren Promotor: Prof. dr. ir. C. Stevens Department of Organic Chemistry, Research Group SynBioC Members of the Examination Committee: Prof. dr. ir. N. De Pauw (Chairman) Prof. dr. J. Marchand-Brynaert Prof. dr. A. Haemers Prof. dr. S. Van Calenbergh Prof. dr. ir. E. Vandamme Prof. dr. ir. R. Verhé Prof. dr. ir. N. De Kimpe Dean: Prof. dr. ir. H. Van Langenhove Rector: Prof. dr. P. Van Cauwenberge IR . KRISTOF MOONEN SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Chemistry Dutch translation of the title: Synthese van 4-fosfono-β-lactamen en aanverwante azaheterocyclische fosfonaten ISBN-Number: 90-5989-129-5 The author and the promotor give the authorisation to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. Woord Vooraf Toen ik op een hete dag in de voorbije zomer dit woord vooraf schreef, stond ik voor één van de laatste horden te nemen in de weg naar het “doctoraat”. Het ideale moment voor een nostalgische terugblik op een zeer fijne periode, hoewel het onzinnig zou zijn te beweren dat alles rozegeur en maneschijn was. En op het einde van de rit komt dan ook het moment waarop je eindelijk een aantal mensen kunt bedanken, omwille van sterk uiteenlopende redenen. -
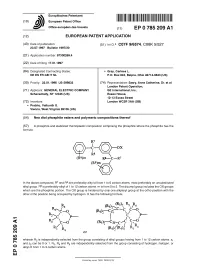
Neo Diol Phosphite Esters and Polymeric Compositions Thereof
^ ^ ^ ^ I ^ ^ ^ ^ ^ ^ II ^ II ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ I ^ European Patent Office Office europeen des brevets EP 0 785 209 A1 EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt ci * C07F 9/6574, C08K 5/527 23.07.1997 Bulletin 1997/30 (21) Application number: 97300269.4 (22) Date of filing: 17.01.1997 (84) Designated Contracting States: • Gray, Carloss L. DE ES FR GB IT NL P.O. Box 843, Belpre, Ohio 45714-0843 (US) (30) Priority: 22.01.1996 US 589832 (74) Representative: Szary, Anne Catherine, Dr. et al London Patent Operation, (71) Applicant: GENERAL ELECTRIC COMPANY GE International, Inc., Schenectady, NY 12345 (US) Essex House, 12-13 Essex Street (72) Inventors: London WC2R 3AA (GB) • Prabhu, Vaikunth S. Vienna, West Virginia 26105 (US) (54) Neo diol phosphite esters and polymeric compositions thereof (57) A phosphite and stabilized themoplastic composition comprising the phosphite where the phosphite has the formula: In the above compound, R7 and R8 are preferably alkyl of from 1 to 6 carbon atoms, most preferably an unsubstituted alkyl group. R9 is preferably alkyl of 1 to 1 2 carbon atoms, m is from 0 to 5. The dicumyl group includes the OX groups which are the phosphite portion. The OX group is hindered by only one alkylaryl group at the ortho position with the other ortho position being occupied by hydrogen. X has the following formula: —r'-^C—C C— O ^c— o (Rsk C p- !/C p- *2 2 C— O (R5)r-CZ2-^c^ O) o (R5)/(R5)2' CM or lO 00 wherein R2 is independently selected from the consisting of alkyl having from 1 to 12 carbon atoms, Is- group groups z-, and z2 can be 0 or 1. -

OJC Vol 31(Special
ORIENTAL JOURNAL OF CHEMISTRY ISSN: 0970-020 X CODEN: OJCHEG An International Open Free Access, Peer Reviewed Research Journal 2015, Vol. 31, (Spl Edn): Month : Oct. www.orientjchem.org Pg. 01-12 Aminomethylenephosphonic Acids Syntheses and Applications (A Review) DIDIER VILLEMIN1 and MOHAMED AMINE DIDI2* 1Laboratoire de Chimie Moléculaire et Thioorganique, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ENSICAEN, 14050 Caen, France. 2Laboratory of Separation and Purification Technology, Tlemcen University, Faculty of Sciences, Department of Chemistry,Box 119, Algeria. *Corresponding author E-mail: [email protected] (Received: March 25, 2015; Accepted: May 10, 2015) http://dx.doi.org/10.13005/ojc/31.Special-Issue1.01 ABSTRACT A review. In this paper, were reviewed the aminomethylenephosphonic acids which have several synthesis routes and many applications as separation of metals and rare earth by solvant extraction, N-C-P in medicinal chemistry, pesticides/herbicides and water-decontamination systems, organometallic complexes: MOF, gas absorption, proton conductivity and Magnetic shielding tensors / aminotris(methylenephosphonates). Key words: Aminomethylphosphonic; solvent extraction; chelate; rare earth metals; pesticides; herbicides. INTRODUCTION O O H H N C N P Aminophosphonic acid can be an OH OH attractive molecule because it can be used in a OH large range of field. The aim of this report is amino acid aminophosphonic acid to summarize synthesis methods of Fig. 1: Similarity between amino acids aminomethylphosphonic acids and their and aminophosphonic acids applications. Most important characteristic of these This similarity gives to aminomethyl- compounds is their N-C-P molecular fragment that phosphonic acid biological applications in broadly defines them as analogues of amino acids, healthcare and agriculture. -

Federal Register/Vol. 72, No. 191/Wednesday, October 3, 2007
Federal Register / Vol. 72, No. 191 / Wednesday, October 3, 2007 / Notices 56347 B. Tips for Preparing Your Comments. under review and the receipt of notices comment, EPA recommends that you When submitting comments, remember of commencement to manufacture those include your name and other contact to: chemicals. This status report, which information in the body of your • Identify the subject by docket covers the period from August 3, 2007 comment and with any disk or CD ROM number and other identifying to September 7, 2007, consists of the you submit. If EPA cannot read your information (subject heading, Federal PMNs and TMEs, both pending or comment due to technical difficulties Register date and page number). expired, and the notices of and cannot contact you for clarification, • Follow directions—The agency may commencement to manufacture a new EPA may not be able to consider your ask you to respond to specific questions chemical that the Agency has received comment. Electronic files should avoid or organize comments by referencing a under TSCA section 5 during this time the use of special characters, any form Code of Federal Regulations (CFR) part period. of encryption, and be free of any defects or section number. DATES: Comments identified by the or viruses. For additional information • Explain why you agree or disagree; specific PMN number or TME number, about EPA’s public docket, visit the EPA suggest alternatives and substitute must be received on or before November Docket Center homepage at http:// language for your requested changes. 2, 2007. www.epa.gov/epahome/dockets.htm. • Docket: All documents in the docket Describe any assumptions and ADDRESSES: Submit your comments, provide any technical information and/ identified by docket identification (ID) are listed in the docket’s index available or data that you used. -

NMR Studies of Phosphorus Ligand Complexes of Silver and Cobalt Steven Mark Socol Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1983 NMR studies of phosphorus ligand complexes of silver and cobalt Steven Mark Socol Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Socol, Steven Mark, "NMR studies of phosphorus ligand complexes of silver and cobalt " (1983). Retrospective Theses and Dissertations. 8962. https://lib.dr.iastate.edu/rtd/8962 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications
materials Review Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications Sophie Wendels †, Thiebault Chavez †, Martin Bonnet, Khalifah A. Salmeia * and Sabyasachi Gaan * Additives and Chemistry Group, Advanced Fibers, Empa, Swiss Federal Laboratories for Materials Science and Technology, Lerchenfeldstrasse 5, 9014 St. Gallen, Switzerland; [email protected] (S.W.); [email protected] (T.C.); [email protected] (M.B.) * Correspondence: [email protected] (K.A.S.); [email protected] (S.G.); Tel.: +41-587-657-038 (K.A.S.); +41-587-657-611 (S.G.) † These two authors contributed equally to this work. Received: 13 May 2017; Accepted: 4 July 2017; Published: 11 July 2017 Abstract: Organophosphorus compounds containing P-C bonds are increasingly developed as flame retardant additives due to their excellent thermal and hydrolytic stability and ease of synthesis. The latest development (since 2010) in organophosphorus flame retardants containing P-C bonds summarized in this review. In this review, we have broadly classified such phosphorus compounds based on the carbon unit linked to the phosphorus atom i.e., could be a part of either an aliphatic or an aromatic unit. We have only considered those published literature where a P-C bond was created as a part of synthetic strategy to make either an intermediate or a final organophosphorus compound with an aim to use it as a flame retardant. General synthetic strategies to create P-C bonds are briefly discussed. Most popular synthetic strategies used for developing P-C containing phosphorus based flame retardants include Michael addition, Michaelis–Arbuzov, Friedels–Crafts and Grignard reactions. -

Aryl Organometallic Complexes Marcella Gagliardo, Dennis J
Reviews G. van Koten et al. DOI: 10.1002/anie.200604290 Synthetic Methods Organic Transformations on s-Aryl Organometallic Complexes Marcella Gagliardo, Dennis J. M. Snelders, Preston A. Chase, Robertus J. M. Klein Gebbink, Gerard P. M. van Klink, and Gerard van Koten* Keywords: aromatic substitution · CÀC coupling · CÀH activation · metallacycles · substituent effects Angewandte Chemie 8558 www.angewandte.org 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Angew. Chem. Int. Ed. 2007, 46, 8558 – 8573 &&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&& &&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&& Take advantage of blue reference links &&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&& &&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&& Angewandte s-Aryl Complexes Chemie This work reviews recent developments in the field of organic trans- From the Contents formations on s-aryl organometallic complexes. The general notion that MÀC s bonds are kinetically labile, highly reactive, and incom- 1. Introduction 8559 patible with typical reaction conditions met in organic synthesis has 2. Regioselective Electrophilic limited the use of these synthetic strategies thus far. However, organic Substitution of transformations on metal-bound s-aryl fragments are being used more s-Bonded Aryl Groups 8560 and more by chemists in both industry and academia. In this Review, -

Molecular Mechanics Based Study on Atomic and Molecular Orbitals of Cobaltocene
id1313906 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com IISenpnteomobrrerg g200aa7 nniicc Volume 2 Issue 3 CCHHEEMMAn IIISnSdiTTan RRJouYYrnal Trade Science Inc. Full Paper ICAIJ, 2(3), 2007 [139-144] Synthesis And Investigation Of The Properties Of L-Tris (Parabenzylidene amino phenyl) Phosphite And Its Metal Carbonyl Derivatives Akbar Raissi Shabari1, Joseph J.Pesek2*, Fariba Raissi Shabari2, Mohamad Mahdavi1 1Teacher Training University of Tehran, Faculty of Chemistry, (IRAN) 2San Jose State University, Chemistry Department, San Jose, CA 95192 (U.S.A.) E-mail : [email protected] Received: 6th February, 2007 ; Accepted: 11th February, 2007 ABSTRACT KEYWORDS The synthesis and investigation of the properties of the Schiff base tris(para- Carbonyl stability; benzylideneaminophenyl)phosphite as well as the preparation in solution at Chiral synthesis catalyst; atmospheric pressure(under N ) of this compounds as a ligand (L) with some Spectroscopic 2 metal carbonyls(mononuclear) having the general formula shown below are characterization. described. M(CO) L ,M=Ni(0),n=4,x=1,2, M=Fe(0),n=5,x=1, M=Cr(0), n-x x n=6, x=1 L=(C H -CH=N-C H -O) P. Elemental analysis as well as infrared, 6 5 6 4 3 ultraviolet-visible and NMR spectroscopic data are also presented. 2007 Trade Science Inc. -INDIA INTRODUCTION important role in organometallic as well as coordina- tion chemistry in general[2,3]. Of particular importance The preparation of L-tris(parabenzylidenea is that phosphite ligands as part of a metal complex minophenyl) phosphate involving the reaction of have been shown to have interesting chiral proper- parabenzylidene aminophenol with PCl proceeds in ties[4,5] and have been used as effective catalysts in 3 refluxing 1,2-C H Cl or toluene with the final prod- enantioselective synthetic processes[6,7].