Blood Agar Plates and Hemolysis Protocols
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Exploring Small-Scale Chemostats to Scale up Microbial Processes: 3-Hydroxypropionic Acid Production in S
Lawrence Berkeley National Laboratory Recent Work Title Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae. Permalink https://escholarship.org/uc/item/43h8866j Journal Microbial cell factories, 18(1) ISSN 1475-2859 Authors Lis, Alicia V Schneider, Konstantin Weber, Jost et al. Publication Date 2019-03-11 DOI 10.1186/s12934-019-1101-5 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Lis et al. Microb Cell Fact (2019) 18:50 https://doi.org/10.1186/s12934-019-1101-5 Microbial Cell Factories RESEARCH Open Access Exploring small-scale chemostats to scale up microbial processes: 3-hydroxypropionic acid production in S. cerevisiae Alicia V. Lis1, Konstantin Schneider1,2, Jost Weber1,3, Jay D. Keasling1,4,5,6,7, Michael Krogh Jensen1 and Tobias Klein1,2* Abstract Background: The physiological characterization of microorganisms provides valuable information for bioprocess development. Chemostat cultivations are a powerful tool for this purpose, as they allow defned changes to one single parameter at a time, which is most commonly the growth rate. The subsequent establishment of a steady state then permits constant variables enabling the acquisition of reproducible data sets for comparing microbial perfor- mance under diferent conditions. We performed physiological characterizations of a 3-hydroxypropionic acid (3-HP) producing Saccharomyces cerevisiae strain in a miniaturized and parallelized chemostat cultivation system. The physi- ological conditions under investigation were various growth rates controlled by diferent nutrient limitations (C, N, P). Based on the cultivation parameters obtained subsequent fed-batch cultivations were designed. Results: We report technical advancements of a small-scale chemostat cultivation system and its applicability for reliable strain screening under diferent physiological conditions, i.e. -

Hemolysis and Venous Thrombosis: Which Link? A
ISSN: 2378-3656 Rkiouak et al. Clin Med Rev Case Rep 2020, 7:329 DOI: 10.23937/2378-3656/1410329 Volume 7 | Issue 11 Clinical Medical Reviews Open Access and Case Reports ORIGINAL RESEARCH Hemolysis and Venous Thrombosis: Which Link? A. Rkiouak, PH.D1, I El Kassimi, MD2, N Sahel, MD2, M Zaizaa, MD2 and Y Sekkach, PhD1 Check for updates Internal Medicine A Department, Mohammed V Military Hospital Medical School of Rabat, Morocco *Corresponding author: Adil Rkiouak, PH.D., Department of Internal Medicine A, Mohammed V Military Hospital, Medical School of Rabat, Morocco, Tel: +212-66-179-44-04 The mechanism of antibody-mediated hemolysis is Abstract via phagocytosis or complement-mediated destruction The association hemolysis and venous thrombosis remains and can occur intravascular or extravascular. The intra- unknown to clinicians, despite our advances in comrehen- sion of pathophysiological bases. vascular mechanisms include direct cellular destruction via lysis, toxins, or trauma; fragmentation and oxida- Haemolysis, which is observed in multiple diseases, can affect all three components of Virchow’s triad. It is not sur- tion. prising that there is a link between haemolytic disorders and Multiple haemolytic disorders produce substantial thrombosis. intravascular haemolysis. Examples, the corpuscular We will try to clarify the main pro-thrombotic mechanisms hemolysis include PNH, extra-corpuscular haemolysis, during hemolysis through 3 clinical observations of deep ve- acquired (autoimmune haemolytic anaemia (AIHA , nous thrombosis in 3 main types of hemolytic pathologies, ) namely a case of paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura (PTT)), as well thrombotic thrombocytopenic purpura and autoimmune as other diseases. These disorders are also associated anemia hemolytic. -

Tryptose Blood Agar Base
Tryptose Blood Agar Base Intended Use Principles of the Procedure Tryptose Blood Agar Base is used with blood in isolating, Tryptose is the source of nitrogen, carbon and amino acids in cultivating and determining the hemolytic reactions of fastidi- Tryptose Blood Agar Base. Beef extract provides additional ous microorganisms. nitrogen. Sodium chloride maintains osmotic balance. Agar is the solidifying agent. Summary and Explanation Investigations of the nutritive properties of tryptose demon- Supplementation with 5-10% blood provides additional growth strated that culture media prepared with this peptone were factors for fastidious microorganisms and is used to determine superior to the meat infusion peptone media previously used hemolytic patterns of bacteria. for the cultivation of Brucella, streptococci, pneumococci, me- Formula ningococci and other fastidious bacteria. Casman1,2 reported Difco™ Tryptose Blood Agar Base that a medium consisting of 2% tryptose, 0.3% beef extract, Approximate Formula* Per Liter 0.5% NaCl, 1.5% agar and 0.03% dextrose equaled fresh beef Tryptose .................................................................... 10.0 g infusion base with respect to growth of organisms. The small Beef Extract ................................................................. 3.0 g amount of carbohydrate was noted to interfere with hemolytic Sodium Chloride ......................................................... 5.0 g Agar ......................................................................... 15.0 g reactions, unless the medium was incubated in an atmosphere *Adjusted and/or supplemented as required to meet performance criteria. of carbon dioxide. Tryptose Blood Agar Base is a nutritious infusion-free basal Directions for Preparation from medium typically supplemented with 5-10% sheep, rabbit or Dehydrated Product horse blood for use in isolating, cultivating and determining 1. Suspend 33 g of the powder in 1 L of purified water. -

Clostridial Septicemia with Intravascular Hemolysis: a Case Report G
Henry Ford Hospital Medical Journal Volume 13 | Number 4 Article 4 12-1965 Clostridial Septicemia With Intravascular Hemolysis: A Case Report G. M. Mastio E. Morfin Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Mastio, G. M. and Morfin, E. (1965) "Clostridial Septicemia With Intravascular Hemolysis: A Case Report," Henry Ford Hospital Medical Bulletin : Vol. 13 : No. 4 , 421-425. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol13/iss4/4 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Bull. Vol. 13, December 1965 CLOSTRIDIAL SEPTICEMIA WITH INTRAVASCULAR HEMOLYSIS A CASE REPORT G. M. MASTIC, M.D. AND E. MORFIN, M.D. In 1871 Bottini' demonstrated the bacterial nature of gas gangrene, but failed to isolate a causal organism. Clostridium perfringens, sometimes known as Clostridium welchii, was discovered independently during 1892 and 1893 by Welch, Frankel, "Veillon and Zuber.^ This organism is a saprophytic inhabitant of the intestinal tract, and may be a harmless saprophyte of the female genital tract occurring in the vagina in 4-6 per cent of pregnant women. Clostridial organisms occur in great numbers and distribution throughout the world. Because of this, they are very common in traumatic wounds. Very few species of Clostridia, however, are pathogenic, and still fewer are capable of producing gas gangrene in man. -

Detection of Acid-Producing Bacteria Nachweis Von Säureproduzierenden Bakterien Détection De Bactéries Produisant Des Acides
(19) TZZ ¥ _T (11) EP 2 443 249 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12Q 1/04 (2006.01) G01N 33/84 (2006.01) 19.11.2014 Bulletin 2014/47 (86) International application number: (21) Application number: 10790013.6 PCT/US2010/038569 (22) Date of filing: 15.06.2010 (87) International publication number: WO 2010/147918 (23.12.2010 Gazette 2010/51) (54) DETECTION OF ACID-PRODUCING BACTERIA NACHWEIS VON SÄUREPRODUZIERENDEN BAKTERIEN DÉTECTION DE BACTÉRIES PRODUISANT DES ACIDES (84) Designated Contracting States: (74) Representative: Isarpatent AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Patent- und Rechtsanwälte GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Friedrichstrasse 31 PL PT RO SE SI SK SM TR 80801 München (DE) (30) Priority: 15.06.2009 US 187107 P (56) References cited: 15.03.2010 US 314140 P US-A- 4 528 269 US-A- 5 098 832 US-A- 5 164 301 US-A- 5 601 998 (43) Date of publication of application: US-A- 5 601 998 US-A- 5 786 167 25.04.2012 Bulletin 2012/17 US-B2- 6 756 225 US-B2- 7 150 977 (73) Proprietor: 3M Innovative Properties Company • DARUKARADHYA J ET AL: "Selective Saint Paul, MN 55133-3427 (US) enumeration of Lactobacillus acidophilus, Bifidobacterium spp., starter lactic acid bacteria (72) Inventors: and non-starter lactic acid bacteria from Cheddar • YOUNG, Robert, F. cheese", INTERNATIONAL DAIRY JOURNAL, Saint Paul, Minnesota 55133-3427 (US) ELSEVIER APPLIED SCIENCE, BARKING, GB, • MACH, Patrick, A. -

Chemostat Culture for Yeast Experimental Evolution
Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press Protocol Chemostat Culture for Yeast Experimental Evolution Celia Payen and Maitreya J. Dunham1 Department of Genome Sciences, University of Washington, Seattle, Washington 98195 Experimental evolution is one approach used to address a broad range of questions related to evolution and adaptation to strong selection pressures. Experimental evolution of diverse microbial and viral systems has routinely been used to study new traits and behaviors and also to dissect mechanisms of rapid evolution. This protocol describes the practical aspects of experimental evolution with yeast grown in chemostats, including the setup of the experiment and sampling methods as well as best laboratory and record-keeping practices. MATERIALS It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol. Reagents Defined minimal medium appropriate for the experiment For examples, see Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Ethanol (95%) Glycerol (20% and 50%; sterile) Yeast strain of interest Equipment Agar plates (appropriate for chosen strain) Chemostat array Assemble the apparatus as described in Miller et al. (2013) and Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Cryo deep-freeze labels Cryogenic vials Culture tubes Cytometer (BD Accuri C6) Glass beads, 4 mm (sterile; for plating yeast cells) Glass cylinder Kimwipes 1Correspondence: [email protected] © 2017 Cold Spring Harbor Laboratory Press Cite this protocol as Cold Spring Harb Protoc; doi:10.1101/pdb.prot089011 559 Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press C. -

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
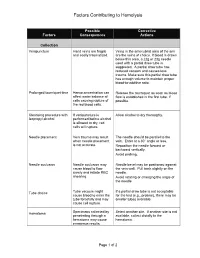
Factors Contributing to Hemolysis
Factors Contributing to Hemolysis Possible Corrective Factors Consequences Actions Collection Venipuncture Hand veins are fragile Veins in the antecubital area of the arm and easily traumatized. are the veins of choice. If blood is drawn below this area, a 22g or 23g needle used with a partial draw tube is suggested. A partial draw tube has reduced vacuum and causes less trauma. Make sure this partial draw tube has enough volume to maintain proper blood-to-additive ratio. Prolonged tourniquet time Hemoconcentration can Release the tourniquet as soon as blood affect water balance of flow is established in the first tube, if cells causing rupture of possible. the red blood cells. Cleansing procedure with If venipuncture is Allow alcohol to dry thoroughly. isopropyl alcohol performed before alcohol is allowed to dry, red cells will rupture. Needle placement Vein trauma may result The needle should be parallel to the when needle placement vein. Enter at a 30° angle or less. is not accurate. Reposition the needle forward or backward vertically. Avoid probing. Needle occlusion Needle occlusion may Needle bevel may be positioned against cause blood to flow the vein wall. Pull back slightly on the slowly and initiate RBC needle. shearing. Avoid rotating or changing the angle of the needle. Tube choice Tube vacuum might If a partial draw tube is not acceptable cause blood to enter the for the test (e.g., protime), there may be tube forcefully and may smaller tubes available. cause cell rupture. Hematoma Specimens collected by Select another site. If another site is not penetrating through a available, collect distally to the hematoma may cause hematoma. -

Maintenance of Bacterial Strains
Maintenance of Bacterial Strains Short-term growth and maintenance The simplest method for obtaining strains is to order them from a microbiological supply company. Presque Isle, Ward, and Carolina Biological (see “Resources” page of the Microbes Web site: www.umsl.edu/~microbes) are economical sources of strains that are commonly used in the classroom. Another possible source is a local college or university that teaches a microbiology lab course. If they have strains already prepared for their class, they will probably be willing to provide you with a culture. This is probably not a reasonable ongoing source of strains, but can be useful in an emergency. For short-term maintenance and use, it is best to streak bacteria on some type of rich agar petri plate, such as nutrient agar. Label and date the plate on the bottom of the plate. Streak from the source culture using good sterile technique. The quadrant streak method (see the article on quadrant streaking) is good for obtaining well-isolated colonies. Incubate the plate only until good colonies have formed. Do not leave the culture in the incubator beyond that time, or the cells will die on the plate. Store the plate in the refrigerator. It will keep for about a month. For class use, it is best to streak a fresh plate 2-3 days before the strain will be needed for class. Use the fresh plate as the source of cultures for the class. For broth cultures for a class, it is best to grow a larger volume culture in a flask (if possible, with shaking) overnight and then dispense aliquots of the master culture into sterile test tubes using a sterile pipette. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

A Suspected Case of Clostridium Perfringens Sepsis With
Uojima et al. Journal of Medical Case Reports (2019) 13:125 https://doi.org/10.1186/s13256-019-2023-x CASE REPORT Open Access A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report Haruki Uojima1,2*, Mie Onoue2, Hisashi Hidaka2, Naohisa Wada2, Yoshiaki Tanaka2, Tomoyoshi Inoue2, Kousuke Kubota2, Takahide Nakazawa2, Akitaka Shibuya2 and Wasaburo Koizumi2 Abstract Introduction: Sepsis due to Clostridium perfringens, one of several clostridial species, is an important cause of massive intravascular hemolysis in patients with underlying malignancies. Chronic liver diseases, immunosuppression, and presence of malignancies were risk factors for Clostridium perfringens sepsis. Therefore, Clostridium perfringens sepsis should always be considered in patients presenting with liver damage after chemo- embolic therapy for hepatocellular carcinoma. This case report focuses on findings characteristic of an intravascular hemolysis due to Clostridium perfringens after transhepatic arterial chemoembolization. Case presentation: An 83-year-old Japanese man presented to our hospital because of a third recurrence of hepatocellular carcinoma. He had nonalcoholic steatohepatitis-related cirrhosis, and underwent radiofrequency ablation and transhepatic arterial chemoembolization therapy for hepatocellular carcinoma of S4/S8 and S2. He had a medical history of pancreatic carcinoma and underwent pylorus-preserving pancreaticoduodenectomy approximately 5 years ago. Because follow-up -

SHEEP BLOOD AGAR - for in Vitro Use Only - Catalogue No
SHEEP BLOOD AGAR - For in vitro use only - Catalogue No. PS58 Our Sheep Blood Agar is a highly nutritious Interpretation of Results medium used for the cultivation and isolation of a variety of microorganisms. Sheep Blood Agar can be used as a primary- Our Sheep Blood Agar is based on the Oxoid plating medium. Primary isolation is performed to formulation; the prepared medium is said to offer separate and isolate organisms present in a sample. improved nutritional value resulting in better growth This separation allows for characterization of and larger colony size as well giving more colony types and may indicate the presence of consistent hemolytic reactions especially among the clinically significant bacteria. When examining streptococci. The base is specifically designed for plates a hand lens or stereoscopic microscope use with sheep blood as horse blood has shown to should be available for examining very small give different and conflicting hemolytic reactions colonies. The different types of colonial when incorporated into other blood agars. morphology appearing on the agar plate should be The nutritional components include pancreatic noted as well as the number of each morphotype digest of casein, neutralized peptone, and yeast present. Hemolysis is also a very useful differential extract, and the addition of sodium chloride characteristic that is best viewed when a bright light provides an osmotically balanced medium for is transmitted from behind the plate. Four different bacterial cells. The addition of 5% defibrinated types of hemolysis can be described: sheep blood allows for the determination of hemolytic reactions, an important differential 1. Alpha-hemolysis ( α) – Partial hemolysis that characteristic.