Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use
Total Page:16
File Type:pdf, Size:1020Kb
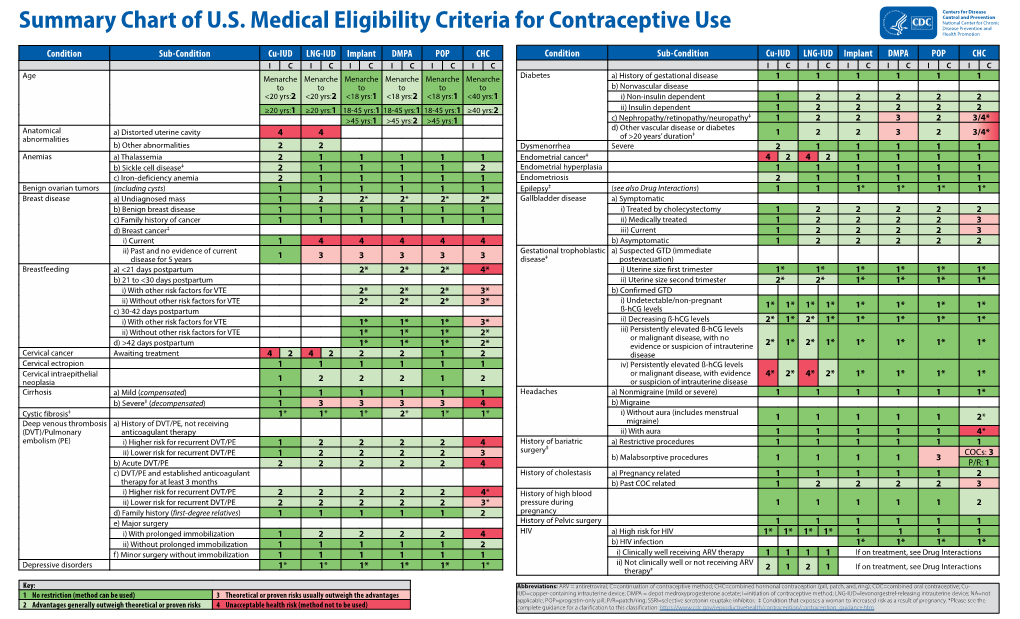
Load more
Recommended publications
-

Regulations Governing the Classification of Medical Devices (Draft)
Regulations Governing the Classification of Medical Devices (Draft) Article 1 These Regulations are enacted pursuant to Paragraph 2, Article 3 of the Medical Devices Act (hereinafter “this Act”). Article 2 Medical devices are classified into the following categories according to their function, intended use, operating instructions, and working principle, depending on the applicable medical specialty: 1. Clinical chemistry and clinical toxicology devices 2. Hematology and pathology devices 3. Immunology and microbiology devices 4. Anesthesiology devices 5. Cardiovascular devices 6. Dental devices 7. Ear, nose, and throat devices 8. Gastroenterology and urology devices 9. General and plastic surgery devices 10. General hospital and personal use devices 11. Neurological devices 12. Obstetrical and gynecological devices 13. Ophthalmic devices 14. Orthopedic devices 15. Physical medicine devices 16. Radiology devices Article 3 Medical devices are classified into the following classes according to their risk level: 1. Class I: Low risk 2. Class II: Medium risk 3. Class III: High risk 1 Article 4 Product items of the medical device classification are specified in the Annex. In addition to rules stated in the Annex, medical devices whose function, intended use, or working principle are special may have their classification determined according to the following rules: 1. If two or more categories, classes, or product items are applicable to the same medical device, the highest class of risk level is assigned. 2. The accessory to a medical device, intended specifically by the manufacturer for use with a particular medical device, is classified the same as the particular medical device, unless otherwise specified in the Annex. 3. -

Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 William A. Rutala, Ph.D., M.P.H.1,2, David J. Weber, M.D., M.P.H.1,2, and the Healthcare Infection Control Practices Advisory Committee (HICPAC)3 1Hospital Epidemiology University of North Carolina Health Care System Chapel Hill, NC 27514 2Division of Infectious Diseases University of North Carolina School of Medicine Chapel Hill, NC 27599-7030 1 Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 3HICPAC Members Robert A. Weinstein, MD (Chair) Cook County Hospital Chicago, IL Jane D. Siegel, MD (Co-Chair) University of Texas Southwestern Medical Center Dallas, TX Michele L. Pearson, MD (Executive Secretary) Centers for Disease Control and Prevention Atlanta, GA Raymond Y.W. Chinn, MD Sharp Memorial Hospital San Diego, CA Alfred DeMaria, Jr, MD Massachusetts Department of Public Health Jamaica Plain, MA James T. Lee, MD, PhD University of Minnesota Minneapolis, MN William A. Rutala, PhD, MPH University of North Carolina Health Care System Chapel Hill, NC William E. Scheckler, MD University of Wisconsin Madison, WI Beth H. Stover, RN Kosair Children’s Hospital Louisville, KY Marjorie A. Underwood, RN, BSN CIC Mt. Diablo Medical Center Concord, CA This guideline discusses use of products by healthcare personnel in healthcare settings such as hospitals, ambulatory care and home care; the recommendations are not intended for consumer use of the products discussed. 2 -

Saudi FDA Products Classification Guidance
Saudi FDA Products Classification Guidance دليل تصنيف المنتجات في الهيئة العامة للغذاء والدواء Version 4.0 Date of publication 21/4/2020 Date of implementation 21/5/2020 1 Saudi FDA Products Classification Guidance Version 4.0 Saudi Food & Drug Authority Kingdom of Saudi Arabia Please review and send your comments and suggestions to [email protected] Please visit SFDA’s website at www.sfda.gov.sa for the latest update 2 Saudi Food & Drug Authority Vision and Mission الرؤية وا لرسالة Vision To be a leading international science-based regulator to protect and promote public health. الرؤي ة أن تكو ن هي ئة رائد ة عالمي ا تستن د إلى أسس علمية لتعزيز وحما ية الصحة العامة Mission Protecting the community through regulations and effective controls to ensure the safety of food, drugs, medical devices, cosmetics, pesticides and feed. الرسالة حماية المجتمع من خﻻل تشريعات ومنظومة رقابية فعالة لضمان سﻻمة الغذاء والدواء واﻷجهزة الطبية ومنتجات التجميل والمبيدات واﻷع ﻻف 3 Document Control Version Author Date Comments 3.0 Products Classification Department 11/04/2019 Final 3.1 Products Classification Department 21/10/2019 Final 4.0 Products Classification Department 21/4/2020 Draft 4 Table of Contents 1. INTRODUCTION ............................................................................................................................... 6 1.1 OBJECTIVES ................................................................................................................................... 6 1.2 BACKGROUND .............................................................................................................................. -

Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices
September 12, 2006 NOTICE Our file number: 06-120629-368 Re: Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices Health Canada is pleased to announce the release of the revised Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices. This guidance document supersedes the January 14, 2000 version of the same document. The Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices is intended to assist manufacturers in confirming the classification of medical device products after application of the Classification Rules for Medical Devices set out in Schedule 1 of the Medical Devices Regulations. This guidance document has been revised to reflect changes to the risk- based classification of particular medical device groups. Specifically, the following major changes have been made to the document: • the reclassification of specific medical device groups; • the removal of redundant information; • the addition of medical device groups; and • the omission of product groups that do not fit the meaning of a medical device as defined in the Food and Drugs Act. To search for a medical device group within the Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices PDF file, go to Edit/Find and enter a keyword, preferred name code or description in the “Find” field. For instance, to verify the risk-based classification of dental burs, enter “bur” or “drill” in the “Find” field. Please note that this guidance document is provided only as a guide and that in the event of any discrepancy between this document and the Classification Rules for Medical Devices described in the Medical Devices Regulations, the latter shall prevail. -

PATIENT EDUCATION SERIES the Intrauterine Device (IUD)
PATIENT EDUCATION SERIES The Intrauterine Device (IUD) What is it? The intrauterine device (IUD) is a small, T-shaped device that is inserted into the uterus. At the end of the T are two plastic strings that hang out of the uterus into the cervix and vagina. These strings are used to remove the IUD. You can check the strings by inserting a finger high up into the vagina. How does it work? There are two types of IUDs: the copper IUD and the hormonal IUD. The hormonal IUD releases a small amount of a hormone called levonorgestrel (a form of progestin). It works by changing the cervical mucus to prevent sperm from reaching the egg. The copper IUD releases a small amount of copper. It works by inactivating sperm. How effective is it? Both types of IUDs are highly effective at preventing pregnancy. Less than 1 woman in 100 will become pregnant within the first year of using an IUD. How long does it last? Both kinds of IUDs give long-term (as in years) protection against pregnancy. Quick Facts About IUDs: How do you get it? You need to have an IUD inserted by a health-care provider. IUDs are among the Types of IUDs most highly effective forms of reversible There are 4 different brands of hormonal IUDs—Mirena, Liletta, Kyleena, and Skyla. They differ in hormone birth control. levels, how long they protect against pregnancy, and the effect that they have on your period. There is only “Reversible” means it’s one brand of copper IUD—Paragard. -

INTRAUTERINE DEVICE (IUD) the Copper IUD Is a T-Shaped Plastic Device Wrapped in Copper Wire
INTRAUTERINE DEVICE (IUD) The copper IUD is a T-shaped plastic device wrapped in copper wire. The hormonal IUD is a T-shaped plastic device that contains 52 milligrams of levonorgestrel (progestin hormone). Both are long-acting reversible contraceptive methods. The copper IUD is effective for 10 years and the hormonal IUD is effective for three to five years. This method must be inserted and removed by a trained provider and does not contain estrogen. MECHANISM OF ACTION METHOD EFFECTIVENESS The IUD works by preventing fertilization. The copper IUD releases copper ions into the uterine cavity which are toxic to the sperm entering the uterus 99.95 IMPLANT 99.8% and fallopian tubes. The hormonal IUD releases 20 99.85 MALE STERILIZATION HORMONAL IUD micrograms of levonorgestrel each day which thickens 99.5 FEMALE STERILIZATION 94 INJECTABLE the cervical mucus and inhibits sperm movement 91 PILL and viability. Both IUDs affect the endometrial lining 82 MALE CONDOM and may prevent implantation of a fertilized egg. 78 WITHDRAWAL However, the primary mechanism of action for the 5 NO METHOD 99.2% COPPER IUD IUD is to prevent fertility earlier in the reproduction process before the egg reaches the uterus. Insufficient evidence exists to support the main mechanism of Note: Method effectiveness is the percent of women NOT action is destruction of the fertilized egg. experiencing pregnancy within the first year of use of each methods It’s a common misconception that IUDs: ...INCREASE THE RISK OF PELVIC ...INCREASE THE RISK OF INFLAMMATORY DISEASE (PID) ECTOPIC PREGNANCY RESEARCH SHOWS the rate of PID is the RESEARCH SHOWS the rate of ectopic same as the general population. -

PEMD-89-10 Medical Devices
Report to the Chairman, Subcommittee on Health and the Environment, Committee on Energy and Commerce, House of Representatives February 1989 : MEDICAL DEVICES FDA’s Implementation of the Medical Device Reporting Regulation ..,___ .-__-.--__-~_-. -- ___- _ ._ - GAO/PEMD-89-10 - Program Evaluation and Methodology Division B-223710 February 17, 1989 The Honorable Henry Waxman Chairman, Subcommittee on Health and the Environment Committee on Energy and Commerce House of Representatives Dear Mr. Chairman: In response to your letter of February 25, 1987, we are submitting this report on FDA'S implementation of the medical device reporting regulation. As agreed with your office, unless you publicly announce the contents of this report earlier, we plan no further distribution of it until 30 days from the date of the report. We will then send copies to interested congressional committees and the Department of Health and Human Services, and we will make copies available to others upon request. This report was prepared under the direction of Michael J. Wargo, Associate Director. Other major contributors to this report are listed in appendix IV. Sincerely yours, Eleanor Chelimsky Assistant Comptroller General Executive Summary ‘ The ability of the Food and Drug Administration (FDA) to protect the Purpose public health has been severely limited by its lack of information on problems associated with medical devices. A previous GAO report (GAO/ PEMD-87-i) found that less than 1 percent of device problems occurring in hospitals were being reported to FDA. However, FDA anticipated that its recently implemented medical device reporting regulation (MDR) would alleviate this and other shortcomings of the agency’s postmarketing sur- veillance system. -

Trainer's GUIDE
TraiNer’s Guide Intrauterine Devices (IUDs) Second Edition Unit 1: An Overview of the IUD Unit 2: Providing Services 1 Key messages 1 Effective IUD counseling 2 Types of IUDs 2 Advise, screen, and select clients 3 Advantages and disadvantages of 3 Loading the TCu 380A inside the the IUD sterile package 4 Indications for using the IUD 4 Performing the steps in IUD insertion 5 Eligibility criteria for IUD use 5 Recommended infection prevention practices 6 Rumors and misconceptions 6 Follow-up management of the IUD 7 Six key steps of counseling client 8 Screen for IUD insertion 7 Minimum clinic requirements and 9 When to insert and remove an IUD recordkeeping tasks necessary for IUD services 10 Insertion and removal procedures 11 Side effects and warning signs Intrauterine Devices (IUDs) Second Edition Trainer’s Guide Cathy Solter Pathfinder International, Watertown MA February, 2008 ©008 Pathfinder International. Any part of this document may be reproduced or adapted to meet local needs without prior permission from Pathfinder International provided Pathfinder International is acknowledged, and the material is made available free of charge or at cost. Please send a copy of all adaptations from this manual to: Technical Services Unit Pathfinder International 9 Galen Street, Suite 17 Watertown, MA 017 INTRAUTERINE DEVICES (IUDs) Acknowledgements The development of Pathfinder International’s training curriculum is an ongoing process and the result of collaboration between many individuals and organizations. The development process of this curriculum began with the privately-funded Reproductive Health Projects (RHPs) in Viet Nam. This manual is based on the adaptation of the Family Planning Course Modules, produced by the Indian Medical Association in collaboration with Development Associates, Inc. -

Contraceptive Options
Contraceptive Options Effectiveness Usage Return to Fertility Protection after use from STD's? Hormonal "The Pill" up to 99% take 1 pill daily 1-3 cycles No at about the Contraceptives (Oral Contraceptives) same time every day * work by preventing "The Patch" up to 99% apply once a 1-3 cycles No release of an egg week for 3 (Ortho Evra) weeks, 4th week off insert into "The Ring” * tend to make periods up to 99% vagina, replace 1-3 cycles No more regular, lighter, (NuvaRing) monthly and less painful "The Shot" > 99% monthly or ovulation may be No (Depo Provera) every 3 months delayed up to 1 year Implantable Progestin-Releasing >99% Skyla 3 years 1 cycle No Intrauterine System Devices (ie - Mirena, Mirena 5 years Skyla or others) * work by preventing release of an egg or by making the uterus Copper Intrauterine >99% 10 years 1 cycle No an "unfriendly" Device (ParaGard IUD) environment Implantable Device >99% 3 years 1 cycle (Nexplanon) No Nonhormonal Male Condom up to 97% new condom N/A Yes with each act of Contraceptives intercourse * prevent pregnancy by Female Condom up to 95% new condom N/A Yes providing a barrier with each act of intercourse against sperm, by interfering with sperm Spermicides 94%, use with N/A No movement, or creating with each act barrier to of intercourse an "unfriendly" increase effectiveness Diaphragm up to 94% with each act N/A minimal environment for sperm of intercourse protection Surgical Sterilization >99 % permanent and permanent No Tubal ligation or irreversible Vasectomy Essure Bedsider.org PAYSON SPANISH FORK Visit - 15 S 1000 E Suite 125 325 W Center CanyonViewWomensCare.com Payson, Utah 84651 Spanish Fork, Utah 84660 for additional information on other Phone : 801-465-2559 Phone : 801-465-2559 interesting health topics. -

Intrauterine Device (Iud) Patient Information
Ottawa County Department of Public Health Family Planning Program INTRAUTERINE DEVICE (IUD) PATIENT INFORMATION GENERAL CONSIDERATIONS ABOUT IUD USE Two types of IUDs available at OCHD: MIRENA® PARAGARD® Contains hormones? Yes No Typical cycle changes Random spotting to no menses Heavier, more crampy menses Approved length of use 5 years 10 years Becomes effective 7 days after insertion Immediately Ability to conceive after May take 1-6 months for cycle Immediately removal to return • Not recommended for short-term use • In general, insertion may be more uncomfortable if you have never been pregnant • Insertion and removal procedures are similar between the two types • Neither one protects against Sexually Transmitted Infections (STIs) including HIV • Higher risk for Pelvic Inflammatory Disease (PID) with both if exposed to an STI • Risk of perforation, embedment , or loss of strings into uterus is similar with both • Both work by preventing fertilization • Risks similar if pregnancy occurs with an IUD in place: ectopic pregnancy, preterm labor, need for IUD removal BEFORE YOUR IUD IS INSERTED • It is very important that you read the manufacturer’s pamphlet or view their information on-line. The information includes the risks and benefits of the IUD you are considering. • Complete our IUD screening form; includes STI risk assessment (possible Chlamydia testing) • Ask questions • No unprotected intercourse from your last menstrual period to insertion - you will need to reschedule your appointment if there’s any chance you’re currently -

Brandname FDA # Approved Dosage Form Company ADVAIR DISKUS
Brandname FDA # Approved Dosage Form Company ADVAIR DISKUS 100/50 021077 2000 POWDER; INHALATION GLAXOSMITHKLINE ALLERNAZE 020120 2000 SPRAY, METERED; NASAL LUPIN ATLANTIS ANDROGEL 021015 2000 GEL; TRANSDERMAL UNIMED PHARMS BIAXIN XL 050775 2000 TABLET, EXTENDED RELEASE; ORAL ABBOTT CONCERTA 021121 2000 TABLET, EXTENDED RELEASE; ORAL ORTHO MCNEIL JANSSEN DEPAKOTE ER 021168 2000 TABLET, EXTENDED RELEASE; ORAL ABBOTT DETROL LA 021228 2000 CAPSULE, EXTENDED RELEASE; ORAL PHARMACIA AND UPJOHN DOXIL 050718 2000 INJECTABLE, LIPOSOMAL; INJECTION ORTHO BIOTECH FLOVENT DISKUS 100 020833 2000 POWDER; INHALATION GLAXOSMITHKLINE GLUCOPHAGE XR 021202 2000 TABLET, EXTENDED RELEASE; ORAL BRISTOL MYERS SQUIBB LESCOL XL 021192 2000 TABLET, EXTENDED RELEASE; ORAL NOVARTIS MIRENA 021225 2000 INTRAUTERINE DEVICE; INTRAUTERINE BAYER NITROSTAT 021134 2000 TABLET; SUBLINGUAL PFIZER PHARMS PAXIL CR 020936 2000 TABLET, EXTENDED RELEASE; ORAL GLAXOSMITHKLINE PULMICORT RESPULES 020929 2000 SUSPENSION; INHALATION ASTRAZENECA QVAR 40 020911 2000 AEROSOL, METERED; INHALATION TEVA GLOBAL TRELSTAR DEPOT 020715 2000 INJECTABLE; INTRAMUSCULAR WATSON LABS VIADUR 021088 2000 IMPLANT; IMPLANTATION ALZA ZYPREXA ZYDIS 021086 2000 TABLET, ORALLY DISINTEGRATING; ORAL LILLY ACCUNEB 020949 2001 SOLUTION; INHALATION DEY ADDERALL XR 10 021303 2001 CAPSULE, EXTENDED RELEASE; ORAL SHIRE ADVICOR 021249 2001 TABLET, EXTENDED RELEASE; ORAL ABBOTT DUONEB 020950 2001 SOLUTION; INHALATION DEY KADIAN 020616 2001 CAPSULE, EXTENDED RELEASE; ORAL ACTAVIS ELIZABETH METADATE CD 021259 2001 CAPSULE, -

IUD (Intrauterine Device)
IUD (intrauterine device) What is an IUD? Intrauterine Devices (IUDs) are small, T-shaped contraceptive devices that provide long term birth control. There are 2 different types: ParaGard (Copper T) - made of flexible plastic and wrapped in copper, this device is hormone free and lasts for 10 years. Mirena - also made of flexible plastic, this device contains the hormone progestin and lasts for 5 years. What is an IUD? An IUD prevents sperm from joining with an egg by affecting how the sperm move. It also thickens cervical mucus to inhibit sperm from passing through & changes the lining of the uterus which prevents egg implantation. IUDs do NOT protect you from STDs What about insertion? IUDs must be inserted by a healthcare provider. Before insertion, you must have a consultation with your provider, be informed of the pros and cons and be screened for gonorrhea and Chlamydia. Your provider may suggest you take ibuprofen before your insertion visit. Some women may experience pain, bleeding and/or dizziness during and after placement. This will normally go away after a few hours. Insertion is more comfortable if performed when you are on your menstrual cycle. In the Exam Room Your vagina will be held open with a speculum and your cervix will be stabilized with a tenaculum. Your uterus will be measured to see if you are a good fit for an IUD. If so, the IUD will be inserted. Many women report feeling discomfort including cramps during insertion. Let your physician know how you are feeling. A sanitary pad will be provided for you since there will likely be some bleeding/spotting afterwards.