Inhalation Therapy in Horses
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Routes of Drug Administration
Routes of Drug Administration Edited by A. T. Florence PhD, DSc, FRSC, FRSE, FRPharmS The School of Pharmacy, University of London, London, UK and E. G. Salole BSc, PhD, MRPharmS Department of Pharmacy, University of Strathclyde, Glasgow, UK WRIGHT London Boston Singapore Sydney Toronto Wellington Wright is an imprint of Butterworth Scientific @l PART OF REED INTERNATIONAL RL.C. All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing it in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright owner except in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 33-34 Alfred Place, London, England WCIE 7DP. Applications for the copyright owner's written permission to reproduce any part of this publication should be addressed to the Publishers. Warning: The doing of an unauthorised act in relation to a copyrigbht work may result in both a civil claim for damages and criminal prosecution. This book is sold subject to the Standard Conditions of Sale of Net Books and may not be re-sold in the UK below the net price given by the Publishers in their current price list. First published 1990 © Butterworth & Co. (Publishers) Ltd, 1990 British Library Cataloguing in Publication Data Routes of drug administration. 1. Medicine. Drug therapy I. Florence, A. T. (Alexander Taylor) II. Salole, E.G. (Eugene G) III. -
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

Priority for Everyone, and Even More So for Those with Chronic Illnesses
Mouth Care: Patient/Caregiver Training Hospice of Cincinnati Mouth Care is a high priority for everyone, and even more so for those with chronic illnesses. Proper mouth care has many benefits including: 1. Increasing appetite 2. Maintaining comfort 3. Clearer speech 4. Easier swallowing 5. Preventing sores and infection. General tips: Mouth care should be performed a minimum of twice daily and more frequently for dry mouth, if breathing through the mouth, or if you are not eating. Mouth care should always be performed after using an inhaler/nebulizer to prevent thrush. Thrush is slightly raised white patches or a rash typically seen on the tongue or inner cheeks. There may be pain with swallowing. Contact your hospice nurse immediately if you suspect thrush. Dentures may no longer fit well. You may consider using them only when eating or when visiting with friends and family. Questions or concerns? Call a hospice nurse at 513-891-7700. Training: Mouth Care. ©2017 Hospice of Cincinnati Hospice of Cincinnati Relieving dry mouth: 1. Increase frequency of mouth care. 2. Encourage sucking on candy, ice chips, popsicles or taking small sips of water to increase saliva. 3. Use of a mouthwash can increase dryness. Talk to your nurse about a proper mouth moisturizer/artificial saliva product. For those with difficulty swallowing: • Raise the head of the bed and support the head with pillows, turn head to one side. • Cover the upper body with a towel to keep the area clean and dry. • Remove dentures prior to mouth care and brush separately. • Use a toothette, which is a sponge-tipped oral swab, to clean the mouth and teeth using a small amount of toothpaste. -

Orally Inhaled & Nasal Drug Products
ORALLY INHALED & NASAL DRUG PRODUCTS: INNOVATIONS FROM MAJOR DELIVERY SYSTEM DEVELOPERS www.ondrugdelivery.com 00349_GF_OnDrugDelivery349_GF_OnDrugDelivery PulmonaryPulmonary NasalNasal NovemberNovember 2010.indd2010.indd 1 330/11/100/11/10 111:32:331:32:33 “Orally Inhaled & Nasal Drug Products: Innovations from Major Delivery CONTENTS System Developers” This edition is one in the ONdrugDelivery series of pub- Innovation in Drug Delivery by Inhalation lications from Frederick Furness Publishing. Each issue focuses on a specific topic within the field of drug deliv- Andrea Leone-Bay, Vice-President, Pharmaceutical ery, and is supported by industry leaders in that field. Development, Dr Robert Baughman, Vice-President, Clinical Pharmacology & Bioanalytics, Mr Chad EDITORIAL CALENDAR 2011: Smutney, Senior Director, Device Technology, February: Prefilled Syringes Mr Joseph Kocinsky, Senior Vice-President, March: Oral Drug Delivery & Advanced Excipients Pharmaceutical Technology Development April: Pulmonary & Nasal Drug Delivery (OINDP) MannKind Corporation 4-7 May: Injectable Drug Delivery (Devices Focus) June: Injectable Drug Delivery (Formulations Focus) Current Innovations in Dry Powder Inhalers September: Prefilled Syringes Richard Sitz, Technical Manager, DPI Technology October: Oral Drug Delivery Platform Leader November: Pulmonary & Nasal Drug Delivery (OINDP) 3M Drug Delivery Systems 10-12 December: Delivering Biologics (Proteins, Peptides & Nucleotides) Pulmonary Delivery & Dry-Powder Inhalers: SUBSCRIPTIONS: Advances in Hard-Capsule -

Injection Technique 1: Administering Drugs Via the Intramuscular Route
Copyright EMAP Publishing 2018 This article is not for distribution except for journal club use Clinical Practice Keywords Intramuscular injection/ Medicine administration/Absorption Practical procedures This article has been Injection technique double-blind peer reviewed Injection technique 1: administering drugs via the intramuscular route rugs administered by the intra- concerns that nurses are still performing Author Eileen Shepherd is clinical editor muscular (IM) route are depos- outdated and ritualistic practice relating to at Nursing Times. ited into vascular muscle site selection, aspirating back on the syringe Dtissue, which allows for rapid (Greenway, 2014) and skin cleansing. Abstract The intramuscular route allows absorption into the circulation (Dough- for rapid absorption of drugs into the erty and Lister, 2015; Ogston-Tuck, 2014). Site selection circulation. Using the correct injection Complications of poorly performed IM Four muscle sites are recommended for IM technique and selecting the correct site injection include: administration: will minimise the risk of complications. l Pain – strategies to reduce this are l Vastus lateris; outlined in Box 1; l Rectus femoris Citation Shepherd E (2018) Injection l Bleeding; l Deltoid; technique 1: administering drugs via l Abscess formation; l Ventrogluteal (Fig 1, Table 1). the intramuscular route. Nursing Times l Cellulitis; Traditionally the dorsogluteal (DG) [online]; 114: 8, 23-25. l Muscle fibrosis; muscle was used for IM injections but this l Injuries to nerves and blood vessels muscle is in close proximity to a major (Small, 2004); blood vessel and nerves, with sciatic nerve l Inadvertent intravenous (IV) access. injury a recognised complication (Small, These complications can be avoided if 2004). -

Hydrogen Peroxide, and This Is the Same Substance That Can Be Purchased in a 32-Ounce Plastic Bottle at Walmart, for 88 Cents, Or at Walgreens for Under a $1.00
An At-Home Treatment That Can Cure Any Virus, Including Coronavirus Originally Conceptualized, circa 1990, by Charles Farr, MD Subsequently Researched and Prescribed by Frank Shallenberger, MD Current Protocol Created by Thomas Levy, MD, JD Although COVID-19, aka coronavirus, is deadly in some select cases, and it can spread rapidly, there is a simple, very inexpensive, and highly effective treatment that can treat and rapidly resolve coronavirus and virtually any other respiratory virus. While different individuals can be expected to have variable degrees of positive response, this intervention can be anticipated to eliminate eventual fatal disease outcomes in all but the most advanced cases. As I hope you will eventually experience, the treatment works for all acute viral infections, and especially well for flu viruses of any variety. In fact, although we are constantly conditioned to not believe in anything “too good to be true,” you will never have to worry about getting a cold or the flu again because you can cure it on your own. The key ingredient in this treatment is common household 3% hydrogen peroxide, and this is the same substance that can be purchased in a 32-ounce plastic bottle at Walmart, for 88 cents, or at Walgreens for under a $1.00. Perhaps you have never heard of hydrogen peroxide therapy, but since the treatment was first championed by Dr. Charles Farr in about 1990, thousands of doctors have used this therapy for decades to conquer infections in many thousands of patients throughout the world. How and Why Hydrogen Peroxide Works Because hydrogen peroxide consists of a water molecule (H2O) with an extra oxygen atom (H2O2), it is this extra oxygen atom that makes it so deadly for viruses. -

Self-Administering a Vitamin B12 Injection
Self-administering a Vitamin B12 injection This is usually given as an intramuscular injection, every 2-3 months. Alternatives to an intramuscular injection are: Oral Vitamin B12 at a dose of at least 1000mcg per day. • Available as tablets or a spray. They can be bought over the counter and available at most health food stores and pharmacies. • Oral Vitamin B12 is not recommended if: - If you have a bowel condition such as inflammatory bowel disease, Coeliac disease, small bowel overgrowth, bile acid malabsorption and short bowel (you will require injections) - You require an initial loading of B12 (soon after diagnosis) It is important to monitor your symptoms if you change to oral B12. If symptoms return, then the oral/sublingual dose can be increased to 2000mcg or you may need to consider starting back on injections. https://www.hollandandbarrett.com/shop/product/betteryou-pure-energy-b12-boost-oral-spray-60099160?skuid=099160 https://www.hollandandbarrett.com/search?query=%20Vitamin%20B12%20Tablets&utm_medium=cpc&utm_source=google&isSearch=true# gclid=EAIaIQobChMIh5nLgOH26AIVxLTtCh0JIA_GEAAYASAAEgJL-fD_BwE&gclsrc=aw.ds Subcutaneous (SC) injection; this is off-licence but still effective. It is considered an easier method of administration. It is how insulin and blood thinning medication are usually self-administered. Equipment needed to self-inject - Prescribed medicine - 1 syringe (2 ml) - 2 needles (1 for drawing up the drug and 1 for administration – you can use the same size needle for both). o For an IM injection; the needle gauge should be 19-25. The needle length is 1- 1 ½ inches (up to 3 inches for larger adults) o For a SC injection; the needle gauge should be 25-27. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

Domoso® Solution
NADA 32-168, Approved by FDA DMSO instilled into the urinary bladder of intact, anesthetized dogs through which an these parameters. DMSO fails to show analgesic or anti-inflammatory activity in certain of In a study designed to evaluate the effects of DOMOSO (dimethyl sulfoxide) Solution at a total enhancement of absorption was demonstrated25. Utilizing a similar technique the transport of these situations, particularly when used by the systemic route or when administered topically daily dose of 100-300 mL administered for a total period of 90 days, no essential or clinically physiologically active insulin across the intact bladder mucosa was demonstrated. Results preceded by an irritant substance. In clinical studies in the horse, it was noted that when meaningful ophthalmological effects were seen in the horse. were judged on a decrease in blood sugar levels over that of controls26. iodine, liniments or other strong irritants were present on the skin from previous therapy and There were no significant variations in glucose, sodium, potassium, SGOT or SGPT DMSO applied, a temporary but marked local reaction would occur. This was due to the In vivo and in vitro methods demonstrated that DMSO enhanced human percutaneous measurements. There were a few fluctuations in hematologic values but no changes appear ability of DMSO to carry these substances into the underlying skin tissues where their irritant ® absorption of various compounds including steroids, vasoconstrictors, antiperspirants and to be drug-related or of significance. DOMOSO SOLUTION actions could be displayed. When DMSO was used clinically, it was applied topically to the dyes, as well as an anthelmintic (thiabendazole) and a skin antiseptic (hexachlorophene)27,28,29,60,61,62. -

Review Article DUOCAP: the CAPSULE in CAPSULE TECHNOLOGY Kanabar Vishvesh B*, Doshi Sumit M, Patel Vipul P Department of Pharmaceutics, School of Pharmacy, R.K
Kanabar Vishvesh B et al. Int. Res. J. Pharm. 2015, 6 (2) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Review Article DUOCAP: THE CAPSULE IN CAPSULE TECHNOLOGY Kanabar Vishvesh B*, Doshi Sumit M, Patel Vipul P Department of Pharmaceutics, School of Pharmacy, R.K. University, Kasturbadham, Rajkot-Bhavnagar Highway, Rajkot, Gujarat, India *Corresponding Author Email: [email protected] Article Received on: 11/12/14 Revised on: 13/01/15 Approved for publication: 18/02/15 DOI: 10.7897/2230-8407.06220 ABSTRACT In this article, the study of never technology of capsule in solid dosage form among all in pharmaceutical dosage forms. This review includes newer trends related to capsule shell, capsule fill material, capsule sealing technique and different capsule systems to achieve modified drug release, encapsulation of various kind of materials and for modified application like mapping of the drug for clinical evaluation Either this done by capsule shell or by dosage filling in capsule dosage forms. This article mostly focuses on advancement of capsule in capsule technology. In this the study is about to reduce the frequency of dosing or to increase effectiveness of the drug by localization at the site of action, reducing the dose required, or providing uniform drug delivery. Keywords: Capsules, Chew caps, Duo caps, Hard Gelatin Capsule, Soft Gelatin Capsule, Vegetarian capsules. INTRODUCTION Since the introduction of Soft Capsule Making Machine in the 1970s, formulations have continually become more popular with The word ‘Capsule’ derived from the Latin word “capsula”, which rapid developments in recent years. This could be illustrated by means a small box or container. -

Efficacy of Systemic Administration of Riboflavin on a Rabbit Model
www.nature.com/scientificreports OPEN Efcacy of systemic administration of ribofavin on a rabbit model of corneal alkali burn Maksym Żuk1*, Ekaterina Lobashova2, Olga Żuk3 & Sławomir Wierzba3 Changes in the barrier mechanisms in the eye should determine the rational route for the administration and dosage of each drug in the treatment of traumatic injuries and other pathologies. The aim of this study was to examine the efcacy of intra-arterial delivery of 14C-ribofavin (as an “indicator”) and compare it with intravenous and intramuscular administration in an animal model of chemical eye burn. 14C-ribofavin (14C-I) was administered by intra-arterial (carotid artery), intravenous (femoral vein) and intramuscular (femoral muscle) routes. The total radioactivity was determined over 2 h in the plasma and structures of the rabbit’s eyes using a scintillation counter. The results of the study show that intravascular administration of 14C-I gives signifcantly higher concentrations of total radioactivity in the blood and is accompanied by a signifcant increase in the permeability of the blood- barrier and barrier in eyes sufering from burns. The highest concentration in the plasma and aqueous humour of the anterior chamber of the eye was observed during the frst hour with the intra-arterial route of administration of 14C-I in either burnt and unburnt eyes. The distribution of total radioactivity in the structures of the eye over the 2 h of the experiment showed a higher level of the drug under intra-arterial administered in the uveal regions, namely: the iris, ciliary body, choroid, retina and also the sclera and cornea. -

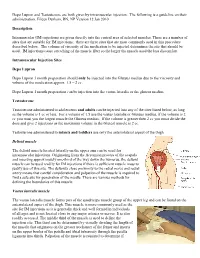
Smoking and Asthma
If you smoke and have asthma, the most important step you can take to improve your health and reduce asthma attacks is to QUIT SMOKING . Tobacco smoke is a major trigger for asthma attacks. Asthmatic breathing tube triggered by smoking Muscles tighten and squeeze tubes Extra mucus forms inside tubes Tubes swell and fill with fluid Asthmatic breathing tube Normal breathing tube Eight hours after your last cigarette, oxygen levels in your No matter blood return to normal. After 72 hours (3 days), breathing tubes relax, lung capacity how long increases, and breathing is easier. After 1 to 9 months, sinus congestion, coughing, fatigue, you smoked, and shortness of breath decrease. Your lungs’ ability to quitting helps clean themselves and handle mucus improves. Chances of lung infections are reduced. you b r e a t h e You’ll save trips to the doctor and ER and save money on asthma medication or possibly not buy it at all. more easily! You’ll save money from no longer buying cigarettes! TOBACCO CESSATION PROGRAM | 2600 TOWER DRIVE, SUITE 216, MONROE Tobacco smoke is the most toxic indoor Quitting smoking is difficult to do alone, air pollutant that triggers asthma. but support is available through your It is worse than dust mites, St. Francis Tobacco Cessation Program. cockroaches, and mold, and people with asthma should not smoke or be around secondhand smoke. Any time you need assistance WHAT HAPPENS WHEN with your cessation journey, need to ask questions YOU QUIT SMOKING or just need to talk, When a person who has asthma quits smok- call us at (318) 966-QUIT.