The Past and Future of Hala (Pandanus Tectorius) in Hawaii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fossil Pollen Record of the Pandanaceae
The Fossil Pollen Record of the Pandanaceae DAVID M. JARZEN Paleobiology Division National Museum of Natural Sciences National Museums of Canada Ottawa, Canada Abstract The fossil record of pollen comparable to the family Pandanaceae and sometimes directly comparable with the extant genus Pandanus extends back to the latest Upper Cretaceous. The family which once had a wide geographic distribution on all continents except Australia, has, since the mid-Tertiary, become restricted to the Old World tropics and subtropics. Introduction The monocotyledon genus Pandanus Rumph. ex L. comprises about 600 species of trees, shrubs and less frequently subshrubs. Several, such as P. epiphyticus Martelli and P. altico/a Holt. and St. John from Malaya and Borneo are truly epiphytic though facultatively so, also occurring on boulders of limestone or sand stone (Stone, 1978). The tree habit is the most common, and such pandans form a conspicuous part of the vegetation of many tropical shorelines. The pandans (or screw-pines) are distributed throughout the palaeotropics, with species occurring on nearly all tropical and marginally subtropical islands of the Pacific, the northern tropical regions of Australia, tropical Southeast Asia, Indonesia, the Philippines, southern India and islands of the Indian Ocean, the Malagasy Republic and East and West Africa (Stone, 1976). The genus does not occur naturally in the neotropics. Map 1 illustrates the overall distribution of the genus, as well as the known fossil pollen reports of Pandanaceae. The pandans are dioecious, with the staminate plants, particularly of the forest species, being less frequently collected than the pistillate plants, due to the brief, ephemeral staminate anthesis. -

Notes on Grasses (Poaceae) in Hawai‘I: 2
Records of the Hawaii Biological Survey for 2009 –2010. Edited by Neal L. Evenhuis & Lucius G. Eldredge. Bishop Museum Occasional Papers 110: 17 –22 (2011) Notes on grasses (Poaceae ) in Hawai‘i : 31. neil snoW (Hawaii Biological survey, Bishop museum, 1525 Bernice street, Honolulu, Hawai‘i, 96817-2704, Usa; email: [email protected] ) & G errit DaViDse (missouri Botanical Garden, P.o. Box 299, st. louis, missouri 63166-0299, Usa; email: [email protected] ) additional new records for the grass family (Poaceae) are reported for Hawai‘i, including five state records, three island records, one corrected island report, and one cultivated species showing signs of naturalization. We also point out minor oversights in need of cor - rection in the Flora of North America Vol. 25 regarding an illustration of the spikelet for Paspalum unispicatum . Herbarium acronyms follow thiers (2010). all cited specimens are housed at the Herbarium Pacificum (BisH) apart from one cited from the missouri Botanical Garden (mo) for Paspalum mandiocanum, and another from the University of Hawai‘i at mānoa (HaW) for Leptochloa dubia . Anthoxanthum odoratum l. New island record this perennial species, which is known by the common name vernalgrass, occurs natu - rally in southern europe but has become widespread elsewhere (allred & Barkworth 2007). of potential concern in Hawai‘i is the aggressive weedy tendency the species has shown along the coast of British columbia, canada, where it is said to be rapidly invad - ing moss-covered bedrock of coastal bluffs, evidently to the exclusion of native species (allred & Barkworth 2007). the species has been recorded previously on kaua‘i, moloka‘i, maui, and Hawai‘i (imada 2008). -

Pandanus Ific Food Leaflet N° Pac 6 ISSN 1018-0966
A publication of the Healthy Pacific Lifestyle Section of the Secretariat of the Pacific Community Pandanus ificifoodileafieiin° Pac i6 ISSN 1018-0966 n parts of the central and northern Pacific, pandanus is a popular food item used in a variety of interesting ways. However, on many other IPacific Islands, pandanus is not well-known as a food. There are many varieties of pandanus, but only In Kiribati, pandanus is called the ‘tree of life’ as it some have edible fruits and nuts. The plants have provides food, shelter and medicine. In the Marshall a distinctive shape and the near-coastal species, Islands, it is called the ‘divine tree’, like coconut, Pandanus tectorius, is found on most Pacific Islands. because of its important role in everyday life. Pandanus The bunches of fruit have many sections called ‘keys’, is also an important staple food in the Federated States which weigh from around 60 to 200 grams each. of Micronesia (FSM), Tuvalu, Tokelau and Papua New (The botanical term for these keys is phalanges, which Guinea. Dried pandanus was once an important food means ‘finger bones’.) People often eat the keys raw, for voyagers on outrigger canoes, enabling seafarers of but the juicy pulp can also be extracted and cooked long ago to survive long journeys. or preserved. The nuts of some varieties are also eaten. In some countries, a number of pandanus varieties are conserved in genebank collections. This leaflet focuses on the Pandanus tectorius species of pandanus. However, other species, such as The pandanus plant plays an important role in Pandanus conoideus and Pandanus jiulianettii, which everyday life in the Pacific. -

Effect of Harvesting Time and Storage on Essential Oil and PEME Content of Pandanus Fascicularis
Journal of Applied Pharmaceutical Science Vol. 7 (10), pp. 185-189, October, 2017 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2017.71027 ISSN 2231-3354 Effect of harvesting time and storage on essential oil and PEME content of Pandanus fascicularis Noohi Nasim1, Jay Krishna Behera2, I. Sriram Sandeep1, Basudeba Kar1, V.V. Ramarao2, Ramesh Srivastava2, Sanghamitra Nayak1, Sujata Mohanty1 ⃰ 1 Centre of Biotechnology, Siksha O Anusandhan University, Bhubaneswar – 751003, India. 2 Fragrances and Flavour Development Centre, Kannauj, MSME, Govt. Of India, India. ABSTRACT ARTICLE INFO Article history: Pandanus fascicularis L. commonly known as kewda is an important aromatic and medicinal plant belonging to Received on: 23/06/2017 the family Pandanaceae. The kewda oil has a characteristic aroma mainly due to the presence of phenyl ethyl Accepted on: 08/09/2017 methyl ether (PEME). In addition, terpinen-4-ol, α-terpineol and p-cymene also contribute to the fragrance of Available online: 30/10/2017 the oil. Owing to its aroma, kewda oil possesses great demand in flavour and fragrance industry. Due to high volatile nature of PEME, the flower harvesting and storage procedures play an important role in quality and Key words: yield of oil. Keeping this in view, the present experiment was carried out where the essential oil was isolated Pandanus fascicularis, from freshly plucked flowers and cold stored kewda flowers (stored at 4 ºC) at 0 hr, 3 hrs and 6 hrs respectively. essential oil, harvesting time, The essential oil was then subjected to GC and GC-MS analysis. The results revealed that the PEME percentage PEME content. -

GALLEY 66 File # 10Em
ECONOMIC BOTANY Wednesday Jan 14 2004 01:03 PM ebot 58_110 Mp_66 Allen Press x DTPro System GALLEY 66 File # 10em CONSERVATION OF USEFUL PLANTS:AN EVALUATION OF LOCAL PRIORITIES FROM TWO INDIGENOUS COMMUNITIES IN EASTERN PANAMA1 SARAH PAULE DALLE AND CATHERINE POTVIN Dalle, Sarah Paule (Department of Plant Science, Macdonald Campus of McGill University, 21111 Lakeshore Dr., Ste-Anne-de-Bellevue, QueÂbec, Canada H9X 3V9; e-mail: sar- [email protected]) and Catherine Potvin (Biology Department, McGill University, 1205 ave Dr. Pen®eld, MontreÂal, QueÂbec, Canada H3A 1B1). CONSERVATION OF USEFUL PLANTS:AN EVALUATION OF LOCAL PRIORITIES FROM TWO INDIGENOUS COMMUNITIES IN EASTERN PANAMA. Economic Botany 58(1):000±000, 2004. On both theoretical and practical grounds, respect for, and inclusion of, local decision-making processes is advocated in conservation, yet little is known about the conservation priorities on local territories. We employed interviews and eco- logical inventories in two villages in order to (1) evaluate the local perception of the conser- vation status of important plant resources; (2) compare patterns of plant use; and (3) compare perceived conservation status with population structure and abundance in the ®eld. One-third of the 35 species examined were perceived to be threatened or declining. These were predom- inantly used locally for construction or sold commercially, but were not necessarily rare in the ®eld. The destructiveness of harvest was the most consistent predictor of conservation status in both villages. Contrasting patterns were found in the two villages for the frequency of plant harvest and the relationship of this variable with conservation status. -

Threatened Endemic Plants of Palau
THREA TENED ENDEMIC PLANTS OF PALAU BIODI VERSITY CONSERVATION LESSONS LEARNED TECHNICAL SERIES 19 BIODIVERSITY CONSERVATION LESSONS LEARNED TECHNICAL SERIES 19 Threatened Endemic Plants of Palau Biodiversity Conservation Lessons Learned Technical Series is published by: Critical Ecosystem Partnership Fund (CEPF) and Conservation International Pacific Islands Program (CI-Pacific) PO Box 2035, Apia, Samoa T: + 685 21593 E: [email protected] W: www.conservation.org The Critical Ecosystem Partnership Fund is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. A fundamental goal is to ensure civil society is engaged in biodiversity conservation. Conservation International Pacific Islands Program. 2013. Biodiversity Conservation Lessons Learned Technical Series 19: Threatened Endemic Plants of Palau. Conservation International, Apia, Samoa Authors: Craig Costion, James Cook University, Australia Design/Production: Joanne Aitken, The Little Design Company, www.thelittledesigncompany.com Photo credits: Craig Costion (unless cited otherwise) Cover photograph: Parkia flowers. © Craig Costion Series Editors: Leilani Duffy, Conservation International Pacific Islands Program Conservation International is a private, non-profit organization exempt from federal income tax under section 501c(3) of the Internal Revenue Code. OUR MISSION Building upon a strong foundation of science, partnership and field demonstration, -

Ecological and Ethnobotanical Facet of 'Kelapa Hutan' (Pandanus Spp
OPEN ACCESS International Journal of Botany ISSN 1811-9700 DOI: 10.3923/ijb.2017.103.114 Research Article Ecological and Ethnobotanical Facet of ‘Kelapa Hutan’ (Pandanus Spp.) and Perspectives Towards its Existence and Benefit 1Krisma Lekitoo, 2Hans Fence Zakeus Peday, 2Novita Panambe and 2Reinardus Liborius Cabuy 1Manokwari Environmental and Forest Research Development Institute, 98314 Manokwari, West Papua Province, Indonesia 2Faculty of Forestry, University of Papua, Gunung Salju St., Amban, 98314 Manokwari, West Papua Province, Indonesia Abstract Background and Objective: Pandanus species are spread across the tropical New Guinea Forest and have long been extracted for food. In this study, the ecological and ethnobotanical aspects of Pandanus spp. were investigated. The objectives of the study were to highlight types of edible Pandanus spp. through their taxonomical characteristics, ecological distribution and fruit properties and to describe how traditional communities manage their existence and traditional values by way of ethnobotany and conservation. Methodology: To identify the potency and distribution of the edible Pandanus, continuous strip-sampling was applied to the sampling plots with an intensity of 5%. Temperature and humidity were measured directly under trees. The edible Pandanus specimens were identified by key experts and identification books. The thermogravimetric and Kjeldahl methods were implemented to identify nutrient contents. A semi-structural interview was implemented, which included questions on the management of Pandanus fruit and its social status among communities. Pearson’s correlation analysis was implemented to identify any relationship between temperature, humidity and fruit productivity. This analysis was performed using R statistical program. Results: Two edible Pandanus species were identified based on each characteristic: Pandanus brosimos Merr. -

Wake Island Grasses Gra Sse S
Wake Island Grasses Gra sse s Common Name Scientific Name Family Status Sandbur Cenchrus echinatus Poaceae Naturalized Swollen Fingergrass Chloris inflata Poaceae Naturalized Bermuda Grass Cynodon dactylon Poaceae Naturalized Beach Wiregrass Dactyloctenium aegyptium Poaceae Naturalized Goosegrass Eleusine indica Poaceae Naturalized Eustachys petraea Poaceae Naturalized Fimbristylis cymosa Poaceae Indigenous Dactyloenium Aegyptium Lepturus repens Poaceae Indigenous Manila grass Zoysia matrella Poaceae Cultivated Cenchrus echinatus Chloris inlfata Fimbristylis cymosa Lepturus repens Zoysia matrella Eustachys petraea Wake Island Weeds Weeds Common Name Scientific Name Family Status Spanish Needle Bidens Alba Asteraceae Naturalized Hairy Spurge Chamaesyce hirta Euphorbiaceae Naturalized Wild Spider Flower Cleome gynandra Capparidaceae Naturalized Purslane Portulaca oleracea Portulaceaceae Naturalized Puncture Vine Tribulus cistoides Zygophyllaceae Indigenous Coat Buttons Tridax procumbens Asteraceae Naturalized Tridax procumbens Uhaloa Waltheria Indica Sterculiacae Indigenous Bidens alba Chamaesyce hirta Cleome gynandra Portulaca oleracea Tribulus cistoides Waltheria indica Wake Island Vines Vines Common Name Scientific Name Family Status Beach Morning Glory Ipomoea pes-caprae Convolvulaceae Indigenous Beach Moonflower Ipomoea violacea Convolvulaceae Indigenous Passion fruit Passiflora foetida Passifloraceae Naturalized Ipomoea violacea Ipomoea pes-caprae Passiflora foetida Wake Island Trees Trees Common Name Scientific Name Family Status -

Ecosystem Services Provided by Bats
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: The Year in Ecology and Conservation Biology Ecosystem services provided by bats Thomas H. Kunz,1 Elizabeth Braun de Torrez,1 Dana Bauer,2 Tatyana Lobova,3 and Theodore H. Fleming4 1Center for Ecology and Conservation Biology, Department of Biology, Boston University, Boston, Massachusetts. 2Department of Geography, Boston University, Boston, Massachusetts. 3Department of Biology, Old Dominion University, Norfolk, Virginia. 4Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, Arizona Address for correspondence: Thomas H. Kunz, Ph.D., Center for Ecology and Conservation Biology, Department of Biology, Boston University, Boston, MA 02215. [email protected] Ecosystem services are the benefits obtained from the environment that increase human well-being. Economic valuation is conducted by measuring the human welfare gains or losses that result from changes in the provision of ecosystem services. Bats have long been postulated to play important roles in arthropod suppression, seed dispersal, and pollination; however, only recently have these ecosystem services begun to be thoroughly evaluated. Here, we review the available literature on the ecological and economic impact of ecosystem services provided by bats. We describe dietary preferences, foraging behaviors, adaptations, and phylogenetic histories of insectivorous, frugivorous, and nectarivorous bats worldwide in the context of their respective ecosystem services. For each trophic ensemble, we discuss the consequences of these ecological interactions on both natural and agricultural systems. Throughout this review, we highlight the research needed to fully determine the ecosystem services in question. Finally, we provide a comprehensive overview of economic valuation of ecosystem services. -
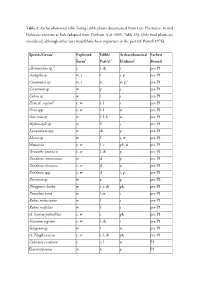
Table 2. Archaeobotanical Table Listing Edible Plants Documented from Late Pleistocene to Mid- Holocene Contexts at Kuk (Adapted from Denham Et Al
Table 2. Archaeobotanical table listing edible plants documented from Late Pleistocene to mid- Holocene contexts at Kuk (adapted from Denham et al. 2003: Table S3). Only food plants are considered, although other uses would have been important in the past (cf. Powell 1976). Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Abelmoschus sp. 5 c l, sh s pre-P1 Acalypha sp. w, t l s, p pre-P1 Castanopsis sp. w, t n w, p? pre-P1 Cerastium sp. w ps pre-P1 Coleus sp. w ls pre-P1 Ficus cf. copiosa5 c, w f, l s pre-P1 Ficus spp. c, w l, f w pre-P1 Garcinia sp. w f, l, b w pre-P1 Hydrocotyle sp. w l? s pre-P1 Lycopodium spp. w sh p pre-P1 Maesa sp. w f s, w pre-P1 Musaceae c, w f, c ph, st pre-P1 Oenanthe javanica c, w l, sh p pre-P1 Pandanus antaresensis w dp pre-P1 Pandanus brosimos c, w d p pre-P1 Pandanus spp. c, w d s, p pre-P1 Parsonsia sp. w np pre-P1 Phragmites karka w l, r, sh ph pre-P1 Pouzolzia hirta w l, st s pre-P1 Rubus moluccanus w fs pre-P1 Rubus rosifolius w fs pre-P1 cf. Setaria palmifolia c, w s ph pre-P1 Solanum nigrum c, w l, sh s pre-P1 Syzygium sp. w f w pre-P1 cf. Zingiberaceae c, w r, l, sh ph pre-P1 Colocasia esculenta c c, l st P1 Elaeocarpaceae w np P1 Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Ipomoea sp. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

Bioactive Components of Pandan's Fruits from Jayawijaya Mountains
IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) e-ISSN: 2319-2402,p- ISSN: 2319-2399.Volume 8, Issue 8 Ver. I (Aug. 2014), PP 01-08 www.iosrjournals.org Bioactive Components of Pandan’s Fruits from Jayawijaya Mountains, Papua, Indonesia Been Kogoya1), Bambang Guritno2) Ariffin2) and Agus Suryanto 2) 1(Graduate School of Agriculture, Department of Agriculture, University of Brawijaya, Malang, East Java, Indonesia) 2(Department of Agriculture, University of Brawijaya, Malang, East Java, Indonesia) Abstract: Five Pandan species, namely Pandanus julianettii, Pandanus iwen, Pandanus brosimos, Pandanus sp.1 (owadak) and Pandanus sp.2 (woromo) from Jaya Wijaya Mountains, Papua have potential bioactive components. This study aimed to identify the bioactive components in Pandan’s fleshy receptacle and seed from five Pandan species. Samples were obtained from fleshy receptacle and seed. Sample were mashed and dried for further proximate, minerals, and vitamins contents analysis. The study revealed that nutritive value compositions of food fiber per 100 grams in fleshy receptacle and seed of Pandanus julianettii were 23% and 12%; those of Pandanus sp.1 (owadak) were 17.59% and 18.38%; those of Pandanus sp.2 (woromo) were 30% and 23%; those of Pandanus iwen were 18% and 30%; those of Pandanus brosimos were 47.75 % and 17.40%. The nutritive value compositions of starch per 100 grams in pandan’s fleshy receptacle and seed of Pandanus julianettii were 23 ppm and 0.24 ppm; those of Pandanus sp.1 (owadak) were 26 ppm and 0.96 ppm; those of Pandanus sp.2 (woromo) were 36 ppm and 18 ppm; those of Pandanus iwen were 21 ppm and 0.21 ppm; those of Pandanus brosimos were 35.88 ppm and 9.67 ppm.