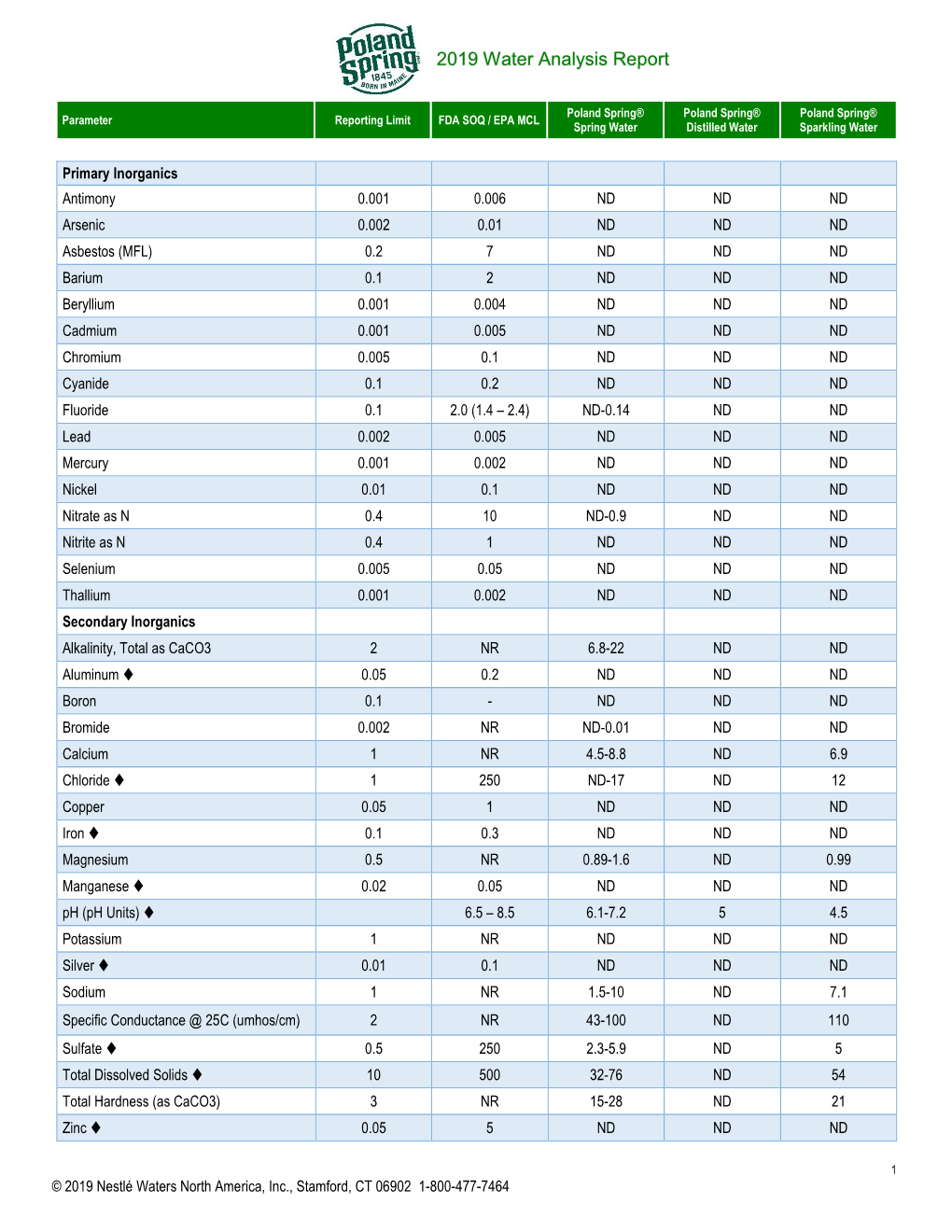
Poland Spring Water Analysis Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arsenic in Drinking Water: What You Need to Know
Arsenic in drinking water: what you need to know Why should I be concerned about arsenic in my drinking water? In Arizona, groundwater can contain arsenic that comes from natural sources, such as rocks and soils, and from human activities such as mining. Short-term exposure to very high doses of arsenic can cause poisoning. Long-term exposure to small doses has been linked to skin changes, cardiovascular effects, and cancer. Whether you are affected depends on how much arsenic you are exposed to and for how long, as well as your sensitivity to arsenic. What level of arsenic in my drinking water is considered safe? The United States Environmental Protection Agency has set the arsenic standard for drinking water at 10 parts per billion (ppb) to protect consumers served by public water systems from the effects of long-term, chronic exposure to arsenic. 10 ppb is roughly equal to 5 teaspoons of ink in an Olympic-sized swimming pool. Read below to learn more about testing and treating your water to reach 10 ppb arsenic or less. How can I learn what level of arsenic is in Arsenic is colorless and tasteless, my drinking water? so you cannot know it is there If you receive water from a municipal or privately-owned unless you test for it. water company, they are required to test your water for arsenic. You should receive an annual water quality report from your water supplier by July 1st of each year. If you drink bottled water, bottled water companies are not required to report results of any water quality testing. -

Sodium Dodecyl Sulfate-Coated Alumina and C18 Cartridge for The
J. Braz. Chem. Soc., Vol. 19, No. 8, 1523-1530, 2008. Printed in Brazil - ©2008 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Sodium Dodecyl Sulfate-Coated Alumina and C18 Cartridge for the Separation and Preconcentration of Cationic Surfactants Prior to their Quantitation by Spectrophotometric Method Mohammad Ali Karimi,*,a,b Reza Behjatmanesh-Ardakani,b Ali Aghaei Goudi b and Sara Zali b aDepartment of Chemistry, Faculty of Science, Payame Noor University (PNU), Sirjan, Iran bDepartment of Chemistry, Faculty of Science, Payame Noor University (PNU), Ardakan, Iran Um novo método de extração em fase sólida foi desenvolvido para separar e pré-concentrar traços de tensoativos catiônicos, tais como, brometo de dodeciltrimetilamônio (DTAB), brometo de cetiltrimetilamônio (CTAB) e cloreto de cetilpiridínio (CPC). Esse método é baseado na sorção do tensoativo aniônico (AS−), dodecilssulfato de sódio (SDS), sobre a superfície de γ-alumina, + enquanto um cartucho C18 é utilizado para a pré-concentração dos tensoativos catiônicos (CS ). O método espectrofotométrico, utilizado para a determinação dos tensoativos catiônicos, baseia-se na competição entre o corante catiônico, azul de metileno (MB+), e o CS+, para associação e formação do complexo SDS. O íon complexo formado (MB+) pode ser quantitativamente substituído pelo CS+, levando a um aumento da absorvância medida em 662 nm. Foram estabelecidas ótimas condições experimentais para a separação, pré-concentração e determinação dos tensoativos catiônicos. Sob essas condições otimizadas, realizou-se a pré-concentração (2×) e os resultados mostraram que a determinação do CPC, DTAB e CTAB poderia ser realizada nas faixas de concentração de 1×10-5-2×10-4, 4×10-5-5×10-4 and 5×10-5-5×10-4 mol L-1, respectivamente. -

Arsenic in Your Well Water What to Do If Your Well Has Too Much Arsenic
Arsenic in Your Well Water What to do if your well has too much arsenic. Switch to bottled water. Finding out your well water has too much arsenic in it may cause you to worry. There are things you can do to protect your family from arsenic. The first thing to do is switch to bottled water for drinking and for making drinks such as coffee, tea, juice, and infant formula. You can use this tipsheet to help you decide what to do next. Call the Maine CDC at 866-292-3474, tollfree in Maine, or 207-287-4311 to speak to an expert about arsenic in your well water. Is there too much arsenic in your water? Arsenic and Health Your test results will have a number then the In most cases, you can protect yourself if you stop letters "ug/L" or "mg/L." These letters are units of drinking water with too much arsenic in it. People measurement, like pounds and ounces. who drink water with too much arsenic for many years are more likely to get cancer. Arsenic can cause skin, Your water has too much arsenic if your test result bladder, and lung cancer. is above 10 ug/L or 0.01 mg/L. It may cause low birthweight and affect brain If your result is higher than these numbers, follow the development in babies if pregnant women drink water advice below. with too much arsenic in it. Arsenic can also affect If your result is between 10 and 50 ug/L or 0.01 and brain development in young children. -

ELGA Veolia Water Guide V6.Pdf
A GUIDE FOR LABORATORIES Pure labwater guide An essential overview of lab water purification applications, monitoring and standards. Dedicated to Discovery 2 Dedicated to Discovery PURE LABWATER GUIDE Inside 3-5 Introduction 6-16 Research and analysis applications 17-20 Clinical diagnostics 21-23 Healthcare 23-47 Water purification overview 48 Glossary 51 Further reading 3 Dedicated to Discovery PURE LABWATER GUIDE The pure labwater guide Introduction The Pure LabWater Guide is an essential resource for individuals who use pure water or wish to learn more about the subject. Providing an overview of water purification requirements, techniques and applications in science and medicine, this educational guide will enable you to choose the correct grade of water and most reliable method of production at an economical cost to both your budget and the environment. CHALLENGES: vary significantly in purity both from There are 5 classes of impurities found IMPURITIES AND one geographical region to another in natural and drinking water: and from season to season. VARIATIONS IN • Suspended particles In today’s laboratories, the availability DRINKING WATER • Dissolved inorganic compounds of pure water is essential, and Water for most laboratory and clinical while domestic consumers consider • Dissolved organic compounds applications is usually purified from tap water to be “pure”, laboratory • Microorganisms & biomolecules drinking water. However, the unique scientists and healthcare professionals ability of water to dissolve (to some regard it as -

Drinking Water Treatment: Distillation Bruce I
® ® University of Nebraska–Lincoln Extension, Institute of Agriculture and Natural Resources Know how. Know now. G1493 (Revised December 2013) Drinking Water Treatment: Distillation Bruce I. Dvorak, Extension Environmental Engineering Specialist Sharon O. Skipton, Extension Water Quality Educator water as it is boiled in the distiller. Such compounds will not Homeowners are increasingly concerned about be completely removed unless another process is used prior contaminants in their water supply that may affect to condensation. See the section in this NebGuide on treat- health or cause taste, odor, or nuisance problems. Dis- ment principles for further discussion of ways distillers may tillation, one of the oldest methods of water treatment, remove VOCs. is an effective method for reducing many impurities The boiling process during distillation generally inacti- found in water. This NebGuide discusses the process vates microorganisms. However, if the distiller is idle for an and related equipment used for household drinking extended period, bacteria can be reintroduced from the outlet water treatment by distillation. spigot and may recontaminate the water. Water Testing Contaminants Removed from Water by Distillation Regardless of which water treatment system is con- Distillation can remove nearly all impurities from sidered, the water first should be tested to determine what water. Compounds removed include sodium, hardness substances are present. Public water systems routinely test compounds such as calcium and magnesium, other dis- for contaminants. Water utilities are required to publish solved solids (including iron and manganese), fluoride, Consumer Confidence Reports (CCRs), which inform con- and nitrate. Operated properly, it effectively inactivates sumers on the source of the water, contaminants present, microorganisms such as bacteria, viruses, and protozoan potential health effects of those contaminants, and methods cysts (though protozoan cysts are not likely to be found in of treatment used by the utility. -

Arsenic in Drinking Water May 2011 DOH 331-167 Revised
Questions & Answers Arsenic in drinking water May 2011 DOH 331-167 Revised What is arsenic and where does it come from? Arsenic occurs naturally in the earth’s crust. Most arsenic in drinking water comes from natural rock formations. As water flows through these formations, it can dissolve arsenic and carry it into underground aquifers, streams, or rivers that may become drinking water supplies. Arsenic also can come from human activities, such as mining or smelting ores that contain arsenic. In the past, it was used in commercial wood preservatives and agricultural chemicals. How can I find out if there’s arsenic in my drinking water supply? Arsenic is tasteless and odorless. Public water systems with at least 25 customers routinely test for arsenic in their water supplies. Your utility issues an annual Consumer Confidence Report that will tell you how much arsenic is in your drinking water. Smaller water systems and private well owners should have their water tested for arsenic by a state-certified lab. We recommend testing twice, preferably in summer and winter to account for seasonal fluctuations. Contact our regional office or your local health department for a list of labs equipped to test for arsenic. Does arsenic affect human health? Yes. Low levels of arsenic in drinking water, soil, air, and food pose a slight health risk. Like most contaminants, the more you are exposed over time, the greater the risk of experiencing health effects. Arsenic health effects include diseases that can affect the cardiovascular system, kidneys, skin, nervous system, or lead to various forms of cancer. -

LIFE WTR Floor Displays Objectives
LIFE WTR Floor Displays Objectives Project objective was to develop a temporary floor stand merchandising vehicle and ceiling hung POS to launch PepsiCo’s premium-priced bottled water called LIFEWTR. To differentiate LIFEWTR from SmartWater, Perrier and others already in the market, PepsiCo developed a label that will change several times during the year and feature different artists that have backgrounds in mediums like graphic design or photography. An important project perimeter was to develop a solution(s) that would showcase the uniquely arted PET bottles. Solution/Details Two solutions were ultimately chosen/placed into retail for this program. One version places complete focus on the product (merchandiser is black and white). The stacked cube structure is clad with packaging graphics, further reinforcing the spectacular visual nature of the sleek product packaging. 360 Shopped Structure & Stacked Cube Structure • 360: Approx. size: 20” x 20” x 69” • Stacked: Approx. size: 15” x 15” x 67” • Litho mounted corrugate for maximum visual impact • Fun & inviGng merchandising structures promote consumer invesGgaon • Stacked: Open sides ease product purchase • 360: Black/White display structure places focus on colorful boNle graphics (product is hero) • Small footprint eases placement in-store • Structures assemble in-store (cubes uGlized in 360 structure are pre-assembled) Production Stacked Cube Structure Mockups 360 Degree Shopped Concept Development Concept Development Concept Development Concept Development Concept Development Concept Development Concept Development Insights In recent years, consumers have been consuming less soda in favor of bottled water, flavored waters and other beverages that are seen as healthier and have fewer artificial ingredients. The launch of internally developed LIFEWTR contributes to a dramatic makeover of PepsiCo’s product offerings (they have vowed that two thirds of their product portfolio will have less than 100 calories per 12oz. -

Bottled Water Logistics and Forecasting" (2013)
University of Arkansas, Fayetteville ScholarWorks@UARK Wal-Mart Sustainability Case Project Supply Chain Management 5-31-2013 Bottled aW ter Logistics and Forecasting Michael Galbreth University of South Carolina Matthew A. Waller University of Arkansas, Fayetteville Christopher Vincent University of Arkansas, Fayetteville David G. Hyatt University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/scmtwscp Part of the Business Administration, Management, and Operations Commons, and the Sustainability Commons Recommended Citation Galbreth, Michael; Waller, Matthew A.; Vincent, Christopher; and Hyatt, David G., "Bottled Water Logistics and Forecasting" (2013). Wal-Mart Sustainability Case Project. 4. http://scholarworks.uark.edu/scmtwscp/4 This Article is brought to you for free and open access by the Supply Chain Management at ScholarWorks@UARK. It has been accepted for inclusion in Wal-Mart Sustainability Case Project by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. The Walmart Sustainability Case Project Date: 05/31/2013 Walmart’s Sustainability Journey: Bottled Water Logistics and Forecasting Eric Smith, regional supply chain manager for Walmart, walked down a concrete aisle between towering shelves of products and pallets. It was a hot day in July 2010, and Smith had made the trip to the backroom of a Conway, Arkansas, Walmart Supercenter to think about water—bottled water, to be specific. The inventory levels had been piling up at an alarming rate at the regional distribution center (DC) in Clarksville, Arkansas, apparently as a result of some overly optimistic forecasting of demand over the July 4 holiday. Smith soon found what he was looking for: a vast section of storage shelves densely packed with pallets of bottled water, ranging from single- serve, 10-ounce bottles to 2.5-gallon jugs. -

ADA Fluoridation Facts 2018
Fluoridation Facts Dedication This 2018 edition of Fluoridation Facts is dedicated to Dr. Ernest Newbrun, respected researcher, esteemed educator, inspiring mentor and tireless advocate for community water fluoridation. About Fluoridation Facts Fluoridation Facts contains answers to frequently asked questions regarding community water fluoridation. A number of these questions are responses to myths and misconceptions advanced by a small faction opposed to water fluoridation. The answers to the questions that appear in Fluoridation Facts are based on generally accepted, peer-reviewed, scientific evidence. They are offered to assist policy makers and the general public in making informed decisions. The answers are supported by over 400 credible scientific articles, as referenced within the document. It is hoped that decision makers will make sound choices based on this body of generally accepted, peer-reviewed science. Acknowledgments This publication was developed by the National Fluoridation Advisory Committee (NFAC) of the American Dental Association (ADA) Council on Advocacy for Access and Prevention (CAAP). NFAC members participating in the development of the publication included Valerie Peckosh, DMD, chair; Robert Crawford, DDS; Jay Kumar, DDS, MPH; Steven Levy, DDS, MPH; E. Angeles Martinez Mier, DDS, MSD, PhD; Howard Pollick, BDS, MPH; Brittany Seymour, DDS, MPH and Leon Stanislav, DDS. Principal CAAP staff contributions to this edition of Fluoridation Facts were made by: Jane S. McGinley, RDH, MBA, Manager, Fluoridation and Preventive Health Activities; Sharon (Sharee) R. Clough, RDH, MS Ed Manager, Preventive Health Activities and Carlos Jones, Coordinator, Action for Dental Health. Other significant staff contributors included Paul O’Connor, Senior Legislative Liaison, Department of State Government Affairs. -

Inorganic Compounds Organic Compounds
BOTTLED WATER INFORMATION Aquafina is purified drinking water that meets and exceeds the requirements set forth by the U.S. Environmental Protection Agency (EPA), the U.S. Food and Drug Administration as well as local regulatory requirements. Our Aquafina plants each conduct on average 320 tests daily, 1950 tests weekly, and − when we include off-site monitoring, 102,000 tests annually to assure the consistent quality of our Aquafina bottled water. A 2019 sample water quality analysis for Aquafina is listed below. Inorganic Compounds Analysis Performed MCL* Results for Purified (mg/L) Finished Product Aluminum 0.2 ND Antimony 0.006 ND Arsenic 0.010 ND Barium 2.0 ND Beryllium 0.004 ND Cadmium 0.005 ND Chloride 250.0 ND Chromium 0.1 ND Copper 1 ND Cyanide (As free cyanide) 0.2 ND Fluoride 4.0 ND Iron 0.3 ND Lead 0.005 ND Manganese 0.05 ND Mercury (Inorganic) 0.002 ND Nickel 0.1 ND Nitrogen (as Nitrate) 10 ND-0.26 Nitrogen (as Nitrite) 1 ND Selenium 0.05 ND Silver 0.1 ND Sulfate 250.0 ND-0.96 Thallium 0.002 ND Zinc 5.0 ND Organic Compounds Analysis Performed MCL* Results for Purified (mg/L) Finished Product Alachlor 0.002 ND Atrazine 0.003 ND Benzene 0.005 ND Benzo(a)pyrene (PAHs) 0.0002 ND Carbofuran 0.04 ND Carbon Tetrachloride 0.005 ND Chlordane 0.002 ND PepsiCo, Inc. Date Updated to Website: June 2019 Page 1 Organic Compounds Cont. Analysis Performed MCL* Results for Purified (mg/L) Finished Product 2,4-D 0.07 ND Dalapon 0.2 ND 1,2-Dibromo-3-chloropropane (DBCP) 0.0002 ND o-Dichlorobenzene 0.6 ND p-Dichlorobenzene 0.075 ND 1,2-Dichloroethane -

An Applied Review of Water Desalination Technologies and an Introduction to Capillary-Driven Desalination
water Article Looking Beyond Energy Efficiency: An Applied Review of Water Desalination Technologies and an Introduction to Capillary-Driven Desalination Seyedsaeid Ahmadvand 1,* , Behrooz Abbasi 1,*, Babak Azarfar 1, Mohammed Elhashimi 2, Xiang Zhang 2 and Bahman Abbasi 2,* 1 Department of Mining and Metallurgical Engineering, University of Nevada, Reno, NV 89557, USA; [email protected] 2 School of Mechanical, Industrial and Manufacturing Engineering, Oregon State University, Bend, OR 97702, USA; [email protected] (M.E.); [email protected] (X.Z.) * Correspondence: [email protected] (S.A.); [email protected] (B.A.); [email protected] (B.A.) Received: 4 March 2019; Accepted: 1 April 2019; Published: 4 April 2019 Abstract: Most notable emerging water desalination technologies and related publications, as examined by the authors, investigate opportunities to increase energy efficiency of the process. In this paper, the authors reason that improving energy efficiency is only one route to produce more cost-effective potable water with fewer emissions. In fact, the grade of energy that is used to desalinate water plays an equally important role in its economic viability and overall emission reduction. This paper provides a critical review of desalination strategies with emphasis on means of using low-grade energy rather than solely focusing on reaching the thermodynamic energy limit. Herein, it is argued that large-scale commercial desalination technologies have by-and-large reached their engineering potential. They are now mostly limited by the fundamental process design rather than process optimization, which has very limited room for improvement without foundational change to the process itself. The conventional approach toward more energy efficient water desalination is to shift from thermal technologies to reverse osmosis (RO). -

Health Effects of Long Term Consumption of Water Low in Calcium, Magnesium Or TDS: Studies from Eastern Europe
(paper presented at the International Symposium on Health Aspects of Calcium and Magnesium in Drinking Water, Baltimore, Maryland, USA, 24-26 April 2006) Health effects of long term consumption of water low in calcium, magnesium or TDS: studies from Eastern Europe Frantisek Kozisek Address: Frantisek Kozisek, M.D., Ph.D,; National Institute of Public Health, Srobarova 48, CZ- 10042 Prague, Czech Republic. Tel.: + 420 267 082 302. Fax: + 420 267 082 271. E-mail: [email protected]. Introduction The relationship between the consumption of soft or low mineralized water and the incidence of some diseases has been discussed in literature since the 1960’s. Some of the recent reviews agree that regular consumption of such water poses a health risk. Nevertheless, critics found some weak points, e.g. the English language bias, as most reviews only included publications in English. This contribution intends to show that in Central and Eastern Europe, multiple authors paid attention to this issue and a lot of studies that have significantly supplemented existing knowledge have been available in several national languages. One of the reasons why desalinisation has, from the very beginning, been considered not only a technical but also public health problem could well be the national methods of managing water supplies. Such surveillance was conducted in the former USSR and other European communist satellites by the Public Health Service; in other words, by medically educated personnel. In the former USSR, the first cue to possible health risks from low mineralized water was provided in the mid 20th century by negative empirical experience gained with the consumption of water from melted snow during polar and climbing expeditions and of distilled water prepared on transoceanic ships.