Kalbitor, INN-Ecallantide
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Complement Peptide C3a Receptor 1 Promotes Optic Nerve Degeneration in DBA/2J Mice Jeffrey M
Harder et al. Journal of Neuroinflammation (2020) 17:336 https://doi.org/10.1186/s12974-020-02011-z RESEARCH Open Access Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice Jeffrey M. Harder1, Pete A. Williams1,2 , Catherine E. Braine1,3, Hongtian S. Yang1, Jocelyn M. Thomas1, Nicole E. Foxworth1, Simon W. M. John1,4,5* and Gareth R. Howell1,6,7* Abstract Background: The risk of glaucoma increases significantly with age and exposure to elevated intraocular pressure, two factors linked with neuroinflammation. The complement cascade is a complex immune process with many bioactive end-products, including mediators of inflammation. Complement cascade activation has been shown in glaucoma patients and models of glaucoma. However, the function of complement-mediated inflammation in glaucoma is largely untested. Here, the complement peptide C3a receptor 1 was genetically disrupted in DBA/2J mice, an ocular hypertensive model of glaucoma, to test its contribution to neurodegeneration. Methods: A null allele of C3ar1 was backcrossed into DBA/2J mice. Development of iris disease, ocular hypertension, optic nerve degeneration, retinal ganglion cell activity, loss of RGCs, and myeloid cell infiltration in C3ar1-deficient and sufficient DBA/2J mice were compared across multiple ages. RNA sequencing was performed on microglia from primary culture to determine global effects of C3ar1 on microglia gene expression. Results: Deficiency in C3ar1 lowered the risk of degeneration in ocular hypertensive mice without affecting intraocular pressure elevation at 10.5 months of age. Differences were found in the percentage of mice affected, but not in individual characteristics of disease progression. -

WO 2016/147053 Al 22 September 2016 (22.09.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/147053 Al 22 September 2016 (22.09.2016) P O P C T (51) International Patent Classification: (71) Applicant: RESVERLOGIX CORP. [CA/CA]; 300, A61K 31/551 (2006.01) A61P 37/02 (2006.01) 4820 Richard Road Sw, Calgary, AB, T3E 6L1 (CA). A61K 31/517 (2006.01) C07D 239/91 (2006.01) (72) Inventors: WASIAK, Sylwia; 431 Whispering Water (21) International Application Number: Trail, Calgary, AB, T3Z 3V1 (CA). KULIKOWSKI, PCT/IB20 16/000443 Ewelina, B.; 31100 Swift Creek Terrace, Calgary, AB, T3Z 0B7 (CA). HALLIDAY, Christopher, R.A.; 403 (22) International Filing Date: 138-18th Avenue SE, Calgary, AB, T2G 5P9 (CA). GIL- 10 March 2016 (10.03.2016) HAM, Dean; 249 Scenic View Close NW, Calgary, AB, (25) Filing Language: English T3L 1Y5 (CA). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, 62/132,572 13 March 2015 (13.03.2015) US BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 62/264,768 8 December 2015 (08. 12.2015) US DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, [Continued on nextpage] (54) Title: COMPOSITIONS AND THERAPEUTIC METHODS FOR THE TREATMENT OF COMPLEMENT-ASSOCIATED DISEASES (57) Abstract: The invention comprises methods of modulating the complement cascade in a mammal and for treating and/or preventing diseases and disorders as sociated with the complement pathway by administering a compound of Formula I or Formula II, such as, for example, 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)- 5,7-dimethoxyquinazolin-4(3H)-one or a pharmaceutically acceptable salt thereof. -

Active Complement Component 1, Q
APD208Hu01 200µg Active Complement Component 1, Q Subcomponent B (C1qB) Organism Species: Homo sapiens (Human) Instruction manual FOR RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES 1th Edition (Apr, 2016) [ PROPERTIES ] Source: Prokaryotic expression. Host: E. coli Residues: Gln28~Ala253 Tags: Two N-terminal Tags, His-tag and SUMO-tag Purity: >94% Endotoxin Level: <1.0EU per 1μg (determined by the LAL method). Buffer Formulation: 20mM Tris, 150mM NaCl, pH8.0, containing 0.05% sarcosyl and 5% trehalose. Applications: Cell culture; Activity Assays. (May be suitable for use in other assays to be determined by the end user.) Predicted isoelectric point: 8.9 Predicted Molecular Mass: 37.5kDa Accurate Molecular Mass: 38kDa as determined by SDS-PAGE reducing conditions. [ USAGE ] Reconstitute in 20mM Tris, 150mM NaCl (pH8.0) to a concentration of 0.1-1.0 mg/mL. Do not vortex. [ STORAGE AND STABILITY ] Storage: Avoid repeated freeze/thaw cycles. Store at 2-8oC for one month. Aliquot and store at -80oC for 12 months. Stability Test: The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37oC for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition. [ SEQUENCE ] [ ACTIVITY ] The complement component 1q (or simply C1q) is a protein complex involved in the complement system, which is part of the innate immune system. C1q together with C1r and C1s form the C1 complex. Antibodies of the adaptive immune system can bind antigen, forming an antigen-antibody complex. -

Pharmacy Prior Authorization Grid ALTCS, and Pharmacy
Please Note: Refer to the other PA grids for applicable covered services that require PA. PA Grids: Medical, Behavioral Health, Pharmacy Prior Authorization Grid ALTCS, and Pharmacy. (Effective Date of Service 1/1/2021) Injectables that require Prior Authorization All chemotherapeutic drugs must be used for FDA-approved indications and/or in accordance with NCCN guidelines *Indicates prior authorization required if billed charges are greater than $400 PA Required HMO 13 HCPCS Short Description (BUCA- Code SNP) 90378 Respiratory Syncytial Virus Immune Globulin Yes C9036 Patisiran Yes C9047 Caplacizumab-yhdp Yes C9061 Teprotumumab-trbw Yes C9063 Eptinezumab-jjmr Yes C9131 Factor VIII antihemophilic factor pegylated-auci Yes C9132 Prothrombin Complex Concentrate (Human), Kcentra Yes C9133 Factor IX (Antihemophilic Factor, Recombinant), Rixibus Yes C9399 Mipomersen (Kynamro) Yes J0129 Abatacept Yes J0135 Adalimumab Yes J0178 Aflibercept Yes J0179 Brolucizumab-dbll, 1 mg Yes J0180 Agalsidase Beta Yes J0205 Alglucerase Yes J0215 Alefacept Yes J0220 Alglucosidase Alfa (Myozyme) Yes J0221 Alglucosidase Alfa (Lumizyme) Yes J0222 Patisiran, 0.1 mg Yes J0223 Givosiran 0.5 mg Yes J0256 Alpha 1-Proteinase Inhibitor Yes J0257 Alpha 1-Proteinase Inhibitor (Glassia) Yes J0275 Alprostadil Urethral Suppository Yes J0490 Belimumab Yes J0517 Benralizumab Yes J0567 Cerliponase alfa Yes J0570 Buprenorphine implant Yes J0584 Burosumab-twza 1 mg Yes J0585 Onabotulinumtoxina (Botox) Yes J0586 Abobotulinumtoxina (Dysport) Yes J0587 Rimabotulinumtoxinb (Myobloc) -

Kalbitor® (Ecallantide)
Kalbitor® (ecallantide) (Subcutaneous) Document Number: MODA-0167 Last Review Date: 10/01/2020 Date of Origin: 08/27/2013 Dates Reviewed: 04/2014, 09/2014, 03/2015, 06/2015, 09/2015, 12/2015, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 06/2017, 09/2017, 12/2017, 03/2018, 06/2018 10/2018, 10/2019, 03/2020, 10/2020 I. Length of Authorization Coverage will be provided for 12 weeks and is eligible for renewal (unless otherwise specified). The cumulative amount of medication(s) the patient has on-hand, indicated for the acute treatment of HAE, will be taken into account when authorizing. The authorization will provide a sufficient quantity in order for the patient to have a cumulative amount of HAE medication on-hand in order to treat up to 4 acute attacks per 4 weeks for the duration of the authorization (unless otherwise specified). II. Dosing Limits A. Quantity Limit (max daily dose) [NDC unit]: Kalbitor 10 mg vial: 24 vials per 28 days B. Max Units (per dose and over time) [HCPCS Unit]: 240 billable units per 28 days III. Initial Approval Criteria 1 Coverage is provided in the following conditions: Patient is at least 12 years of age; AND Universal Criteria 1,13,18 Must be prescribed by, or in consultation with, a specialist in: allergy, immunology, hematology, pulmonology, or medical genetics; AND Confirmation the patient is avoiding the following possible triggers for HAE attacks: o Estrogen-containing oral contraceptive agents AND hormone replacement therapy; AND o Antihypertensive agents containing ACE inhibitors; AND o Dipeptidyl peptidase IV (DPP-IV) inhibitors (e.g., sitagliptin); AND o Neprilysin inhibitors (e.g., sacubitril) Moda Health Plan, Inc. -

High-Throughput Proteomic Profiling of the Fish Liver Following Bacterial
Causey et al. BMC Genomics (2018) 19:719 https://doi.org/10.1186/s12864-018-5092-0 RESEARCH ARTICLE Open Access High-throughput proteomic profiling of the fish liver following bacterial infection Dwight R Causey1, Moritz A N Pohl1, David A Stead2, Samuel A M Martin1, Christopher J Secombes1 and Daniel J Macqueen1* Abstract Background: High-throughput proteomics was used to determine the role of the fish liver in defense responses to bacterial infection. This was done using a rainbow trout (Oncorhynchus mykiss) model following infection with Aeromonas salmonicida, the causative agent of furunculosis. The vertebrate liver has multifaceted functions in innate immunity, metabolism, and growth; we hypothesize this tissue serves a dual role in supporting host defense in parallel to metabolic adjustments that promote effectiveimmunefunction.Whilepaststudieshavereported mRNA responses to A. salmonicida in salmonids, the impact of bacterial infection on the liver proteome remains uncharacterized in fish. Results: Rainbow trout were injected with A. salmonicida or PBS (control) and liver extracted 48 h later for analysis on a hybrid quadrupole-Orbitrap mass spectrometer. A label-free method was used for protein abundance profiling, which revealed a strong innate immune response along with evidence to support parallel rewiring of metabolic and growth systems. 3076 proteins were initially identified against all proteins (n = 71,293 RefSeq proteins) annotated in a single high-quality rainbow trout reference genome, of which 2433 were maintained for analysis post-quality filtering. Among the 2433 proteins, 109 showed significant differential abundance following A. salmonicida challenge, including many upregulated complement system and acute phase response proteins, in addition to molecules with putative functions that may support metabolic re-adjustments. -

The Use of Radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor Deficiency
Avances en Biomedicina ISSN: 2477-9369 ISSN: 2244-7881 [email protected] Universidad de los Andes Venezuela The use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Lara de la Rosa, María del Pilar; Conde Alcañiz, Amparo; Moreno Ramírez, David; Illescas Vacas, Ana; Guardia Martínez, Pedro The use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Avances en Biomedicina, vol. 7, no. 2, 2018 Universidad de los Andes, Venezuela Available in: https://www.redalyc.org/articulo.oa?id=331359393006 PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Casos Clínicos e use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Uso de radioterapia en Angioedema hereditario por déficit de C1 inhibidor tipo I María del Pilar Lara de la Rosa [email protected] University Hospital Virgen Macarena, España Amparo Conde Alcañiz University Hospital Virgen Macarena, España David Moreno Ramírez University Hospital Virgen Macarena, España Ana Illescas Vacas University Hospital Virgen Macarena, España Pedro Guardia Martínez University Hospital Virgen Macarena, España Avances en Biomedicina, vol. 7, no. 2, 2018 Universidad de los Andes, Venezuela Received: 27 February 2018 Accepted: 21 June 2018 Abstract: We present a clinical case of a 72 year old man with Hereditary Angioedema Type 1. It´s a rare, potentially fatal disease, especially due to causing episodes of Redalyc: https://www.redalyc.org/ laryngeal angioedema. He has a past medical history of lip squamous-cell skin cancer, articulo.oa?id=331359393006 which is currently relapsing, with lateral margins of the surgical resection affected requiring treatment with local radiotherapy. -

Deficiency of Complement Component 5 Ameliorates Glaucoma in DBA/2J Mice
Rowan University Rowan Digital Works Faculty Scholarship for the College of Science & Mathematics College of Science & Mathematics 6-27-2013 Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice Gareth R. Howell Ileana Soto Reyes Rowan University, [email protected] Margaret Ryan Leah C. Graham Richard S. Smith See next page for additional authors Follow this and additional works at: https://rdw.rowan.edu/csm_facpub Part of the Neuroscience and Neurobiology Commons Recommended Citation Howell, G. R., Soto, I., Ryan, M., Graham, L. C., Smith, R. S., & John, S. W. (2013). Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. Journal of Neuroinflammation, 10, 76-2094-10-76. This Article is brought to you for free and open access by the College of Science & Mathematics at Rowan Digital Works. It has been accepted for inclusion in Faculty Scholarship for the College of Science & Mathematics by an authorized administrator of Rowan Digital Works. Authors Gareth R. Howell, Ileana Soto Reyes, Margaret Ryan, Leah C. Graham, Richard S. Smith, and Simon W.M. John This article is available at Rowan Digital Works: https://rdw.rowan.edu/csm_facpub/24 Howell et al. Journal of Neuroinflammation 2013, 10:76 JOURNAL OF http://www.jneuroinflammation.com/content/10/1/76 NEUROINFLAMMATION RESEARCH Open Access Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice Gareth R Howell1*†, Ileana Soto1†, Margaret Ryan1, Leah C Graham1, Richard S Smith1 and Simon WM John1,2,3* Abstract Background: Glaucoma is an age-related neurodegenerative disorder involving the loss of retinal ganglion cells (RGCs), which results in blindness. -
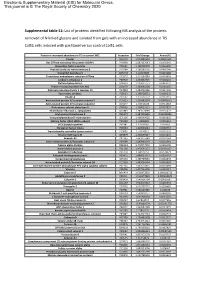
Supplemental Table S1
Electronic Supplementary Material (ESI) for Molecular Omics. This journal is © The Royal Society of Chemistry 2020 Supplemental table S1: List of proteins identified following MS analysis of the proteins removed of N-linked glycans and isolated from gels with an increased abundance in TIS Cal51 cells induced with paclitaxel versus control Cal51 cells. Protein in increased abundance in TIS vs control WCL Accession Fold Change Anova (P) Plectin Q15149 1.073855593 0.00691631 Ras GTPase-activating-like protein IQGAP1 P46940 1.087337643 0.0176342 Elongation factor1-gamma P26641 1.138709703 0.0116496 Peptidyl-prolyl cis-transisomerase B P23284 1.188383105 0.0436246 Dipeptidyl peptidase 3 Q9NY33 1.20163605 0.0215448 Transitional endoplasmic reticulum ATPase P55072 1.214194884 0.0449691 Carbonic anhydrase 2 P00918 1.232852325 0.0158141 Clathrin heavy chain 1 Q00610 1.239621773 0.0463237 Protein transport protein Sec 31A O94979 1.263565104 0.0284155 Aldo-ketoreductase family 1 member C1 Q04828 1.282092186 0.0324406 Spermidine synthase P19623 1.298728621 0.0196232 Plastin-3 P13797 1.310756772 0.0161319 Actin-related protein 2/3 complex subunit 5 O15511 1.333483524 0.00476923 Actin-related protein 2/3 complex subunit 2 O15144 1.35416168 0.0411018 Proteasome subunit alpha type-5 P28066 1.358015551 0.0337657 Thioredoxin reductase 1, cytoplasmic Q16881 1.383670089 0.0235472 Acyl-protein thioesterase 2 O95372 1.387415589 0.00233899 Isoaspartylpeptidase/L-asparaginase Q7L266 1.408149002 0.0319602 Splicing factor U2AF 65kDa subunit P26368 1.41489991 0.0256619 -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Kalbitor® (Ecallantide)
Kalbitor® (ecallantide) (Subcutaneous) Document Number: IC-0167 Last Review Date: 03/03/2020 Date of Origin: 08/27/2013 Dates Reviewed: 04/2014, 09/2014, 03/2015, 06/2015, 09/2015, 12/2015, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 06/2017, 09/2017, 12/2017, 03/2018, 06/2018 10/2018, 10/2019, 03/2020 I. Length of Authorization Coverage will be provided for 12 weeks and is eligible for renewal (unless otherwise specified). The cumulative amount of medication(s) the patient has on-hand, indicated for the acute treatment of HAE, will be taken into account when authorizing. The authorization will provide a sufficient quantity in order for the patient to have a cumulative amount of HAE medication on-hand in order to treat up to 4 acute attacks per 4 weeks for the duration of the authorization (unless otherwise specified). II. Dosing Limits A. Quantity Limit (max daily dose) [NDC unit]: − Kalbitor 10 mg vial: 24 vials per 28 days B. Max Units (per dose and over time) [HCPCS Unit]: • 240 billable units per 28 days 1-15 III. Initial Approval Criteria Coverage is provided in the following conditions: Universal Criteria: • Must be prescribed by, or in consultation with, a specialist in: allergy, immunology, hematology, pulmonology, or medical genetics; AND • Confirmation the patient is avoiding the following possible triggers for HAE attacks: o Estrogen-containing oral contraceptive agents AND hormone replacement therapy; AND o Antihypertensive agents containing ACE inhibitors; AND Treatment of acute attacks of Hereditary Angioedema (HAE) † • Patient must be at least 12 years of age; AND Proprietary & Confidential © 2020 Magellan Health, Inc. -

In Kidney Injury
antioxidants Review Regulation of Complement Activation by Heme Oxygenase-1 (HO-1) in Kidney Injury Maria G. Detsika 1,* and Elias A. Lianos 2,3 1 First Department of Critical Care Medicine & Pulmonary Services, GP Livanos and M. Simou Laboratories, National & Kapodistrian University of Athens, Medical School, Evangelismos Hospital, 10675 Athens, Greece 2 Thorax Foundation, Research Center of Intensive Care and Emergency Thoracic Medicine, 10675 Athens, Greece; [email protected] 3 Veterans Affairs Medical Center and Virginia Tech, Carilion School of Medicine, 1970 Roanoke Blvd, Salem, VA 24153, USA * Correspondence: [email protected]; Tel.: +30-210-723552; Fax: +30-210-7239127 Abstract: Heme oxygenase is a cytoprotective enzyme with strong antioxidant and anti-apoptotic properties. Its cytoprotective role is mainly attributed to its enzymatic activity, which involves the degradation of heme to biliverdin with simultaneous release of carbon monoxide (CO). Recent studies uncovered a new cytoprotective role for heme oxygenase-1 (HO-1) by identifying a regulatory role on the complement control protein decay-accelerating factor. This is a key complement regulatory protein preventing dysregulation or overactivation of complement cascades that can cause kidney injury. Cell-specific targeting of HO-1 induction may, therefore, be a novel approach to attenuate complement-dependent forms of kidney disease. Keywords: heme; heme oxygenase-1 (HO-1); complement; kidney injury 1. Introduction Citation: Detsika, M.G.; Lianos, E.A. Although the role of heme, in various cellular processes, such as gene transcription Regulation of Complement and translation and cellular differentiation, proliferation, and apoptosis, has been known Activation by Heme Oxygenase-1 for decades, the role of the heme-degrading enzyme heme oxygenase-1 (HO-1) only gained (HO-1) in Kidney Injury.