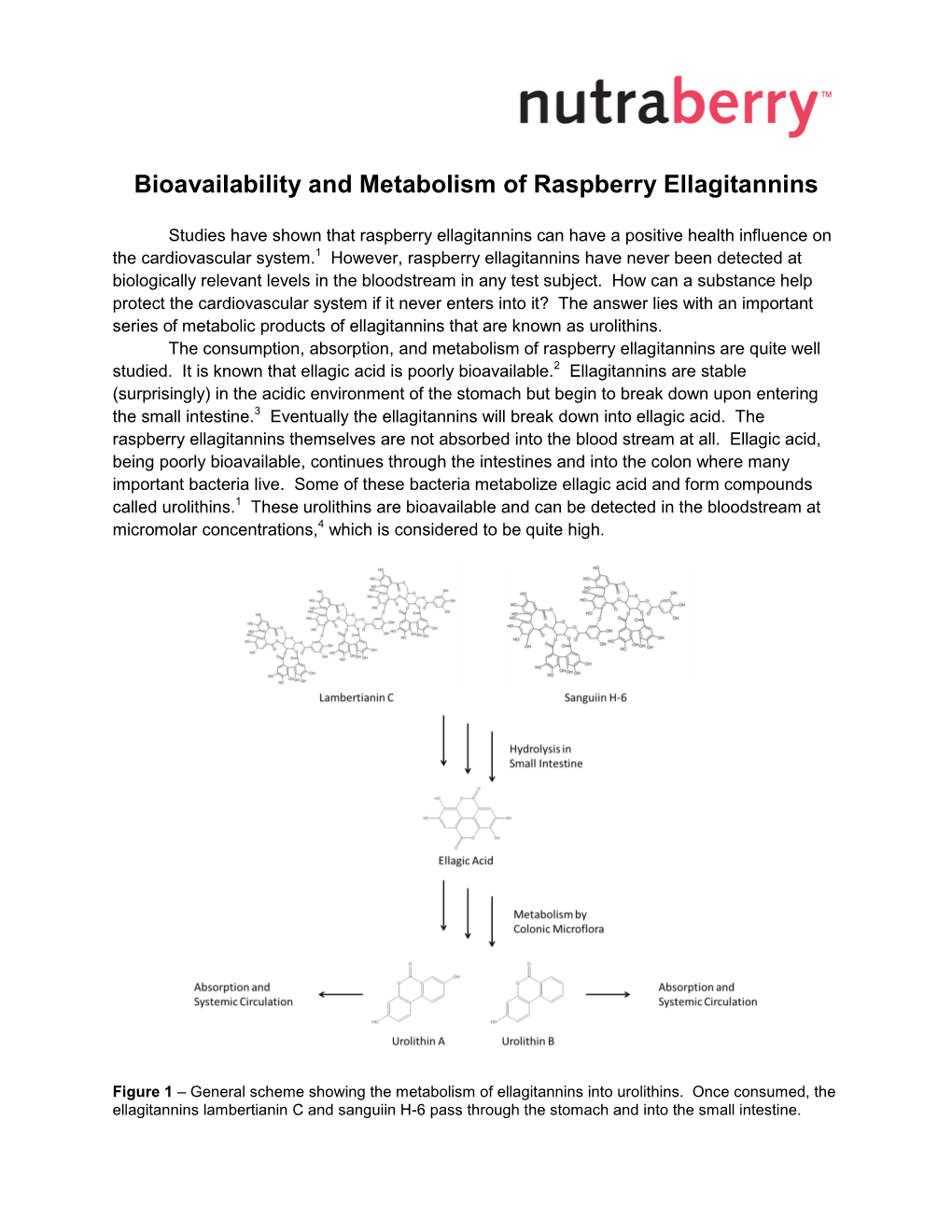
Bioavailability and Metabolism of Raspberry Ellagitannins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ellagitannins with a Glucopyranose Core Have Higher Affinity to Proteins Than Acyclic Ellagitannins by Isothermal Titration Calorimetry
Ellagitannins with a glucopyranose core have higher affinity to proteins than acyclic ellagitannins by isothermal titration calorimetry Article Supplemental Material Karonen, M., Oraviita, M., Mueller-Harvey, I., Salminen, J.-P. and Green, R. J. (2019) Ellagitannins with a glucopyranose core have higher affinity to proteins than acyclic ellagitannins by isothermal titration calorimetry. Journal of Agricultural and Food Chemistry, 67 (46). pp. 12730-12740. ISSN 0021-8561 doi: https://doi.org/10.1021/acs.jafc.9b04353 Available at http://centaur.reading.ac.uk/87023/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1021/acs.jafc.9b04353 Publisher: American Chemical Society All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Supporting Information Ellagitannins with a Glucopyranose Core Have Higher Affinity to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry Maarit Karonen*,†, Marianne Oraviita†, Irene Mueller-Harvey‡, Juha-Pekka Salminen†, and Rebecca J. Green*,§ †Natural Chemistry Research Group, Department of Chemistry, University of Turku, Vatselankatu 2, Turun Yliopisto, Turku FI-20014, Finland ‡School of Agriculture, Policy and Development, University of Reading, Earley Gate, P.O. Box 236, Reading RG6 6AT, United Kingdom §School of Chemistry, Food and Pharmacy, University of Reading, Whiteknights, P.O. -

In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: a Case of Padma Hepatenr Formulation
Article In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepatenr Formulation Daniil N. Olennikov 1,*, Nina I. Kashchenko 1,: and Nadezhda K. Chirikova 2,: Received: 28 August 2015 ; Accepted: 30 September 2015 ; Published: 13 October 2015 1 Laboratory of Medical and Biological Research, Institute of General and Experimental Biology, Siberian Division, Russian Academy of Science, Sakh’yanovoy Street 6, Ulan-Ude 670-047, Russia; [email protected] 2 Department of Biochemistry and Biotechnology, North-Eastern Federal University, 58 Belinsky Street, Yakutsk 677-027, Russian; [email protected] * Correspondence: [email protected]; Tel.: +7-9021-600-627; Fax: +7-3012-434-243 : These authors contributed equally to this work. Abstract: Chebulic ellagitannins (ChET) are plant-derived polyphenols containing chebulic acid subunits, possessing a wide spectrum of biological activities that might contribute to health benefits in humans. The herbal formulation Padma Hepaten containing ChETs as the main phenolics, is used as a hepatoprotective remedy. In the present study, an in vitro dynamic model simulating gastrointestinal digestion, including dialysability, was applied to estimate the bioaccessibility of the main phenolics of Padma Hepaten. Results indicated that phenolic release was mainly achieved during the gastric phase (recovery 59.38%–97.04%), with a slight further release during intestinal digestion. Dialysis experiments showed that dialysable phenolics were 64.11% and 22.93%–26.05% of their native concentrations, respectively, for gallic acid/simple gallate esters and ellagitanins/ellagic acid, in contrast to 20.67% and 28.37%–55.35% for the same groups in the non-dialyzed part of the intestinal media. -

Table 2 of Supporting Information
Electronic Supplementary Material (ESI) for Food & Function. This journal is © The Royal Society of Chemistry 2016 Table S1 Supplementary Information. Optimized SRM conditions used for quantification for the analysis of phenolic compounds by UPLC-MS/MS. Quantification Phenolic compound MW Collision energy Standard used for quantification SRM Cone voltage (v) (eV) Catechol 110 108.9 90.9 40 15 Catechol Catechol sulfate 190 189 109 20 15 Catechol Catechol glucuronide 286 285 123 40 15 Catechol Pyrogallol sulfate 206 205 125 20 15 Catechol Methyl pyrogallol sulfate 220 219 124 20 25 Catechol Pyrogallol glucuronide 302 301 125 20 10 Catechol Pyrogallol glucuronide-sulfate 382 381 125 20 10 Catechol p-Hydroxybenzoic acid 138 137 93 30 15 p-Hydroxybenzoic acid Hydroxybenzoic acid 138 137 93 30 15 p-Hydroxybenzoic acid Protocatechuic acid 154 153 109 40 15 Protocatechuic acid Gallic acid 170 169 125 35 10 Gallic acid Gallic acid hexoside 332 331 169 40 15 Gallic acid Mono-O-galloylquinic acid 344 343 191 40 15 Gallic acid Di-O-galloylquinic acid 496 495 191 40 25 Gallic acid Tri-O-galloylquinic acid 648 647 495 40 15 Gallic acid Tetra-O-galloylquinic acid 630 629 477 40 15 Gallic acid Mono-O-galloylshikimic acid 326 325 169 40 20 Gallic acid Di-O-galloylshikimic acid 478 477 325 40 20 Gallic acid Gallic acid sulphate 250 249 169 35 15 Gallic acid Gallic acid glucuronide 346 345 169 35 15 Gallic acid Syringic acid 198 197 182 30 10 Syringic acid Ellagic acid arabinoside 434 433 300 40 30 Ellagic acid Ellagic acid glucuronide -

Concentrations of Blood Serum and Urinal Ellagitannin Metabolites Depend Largely on the Post-Intake Time and Duration of Strawberry Phenolics Ingestion in Rats
Pol. J. Food Nutr. Sci., 2019, Vol. 69, No. 4, pp. 379–386 DOI: 10.31883/pjfns/111866 http://journal.pan.olsztyn.pl Original article Section: Nutritional Research Concentrations of Blood Serum and Urinal Ellagitannin Metabolites Depend Largely on the Post-Intake Time and Duration of Strawberry Phenolics Ingestion in Rats Ewa Żary-Sikorska1*, Monika Kosmala2, Joanna Milala2, Bartosz Fotschki3, Katarzyna Ognik4, Jerzy Juśkiewicz3 1Department of Microbiology and Food Technology, Faculty of Agriculture and Biotechnology University of Science and Technology, Kaliskiego 7, 85–796 Bydgoszcz, Poland 2Institute of Food Technology and Analysis, Łódź University of Technology, Stefanowskiego 4/10, 90–924 Łódź, Poland 3Department of Biological Functions of Food, Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences, Tuwima 10, 10–748 Olsztyn, Poland 4Department of Biochemistry and Toxicology, Faculty of Biology, Animal Sciences and Bioeconomy, University of Life Sciences, Akademicka 13, 20–950 Lublin, Poland Key words: strawberry, ellagitannins, metabolites, urine, serum, rat The different duration of a strawberry phenolic fraction intake and different post-intake time were experimental factors affecting the concentrations of ellagitannin metabolites in the urine and blood serum of rats. For four days, the animals were gavaged once a day as follows: group C (water, days 1–4), group F1–4 (fraction, days 1–4), group F1–3 (fraction, days 1–3; water, day 4), group F1–2 (fraction, days 1, 2; water, days 3, 4), group F3–4 (water, days 1, 2; fraction, days 3, 4), and group F4 (water, days 1–3; and fraction, day 4). The daily dosage of the fraction gavaged to one rat was 20 mg/kg of body weight. -

Universidade Federal Do Rio De Janeiro Kim Ohanna
UNIVERSIDADE FEDERAL DO RIO DE JANEIRO KIM OHANNA PIMENTA INADA EFFECT OF TECHNOLOGICAL PROCESSES ON PHENOLIC COMPOUNDS CONTENTS OF JABUTICABA (MYRCIARIA JABOTICABA) PEEL AND SEED AND INVESTIGATION OF THEIR ELLAGITANNINS METABOLISM IN HUMANS. RIO DE JANEIRO 2018 Kim Ohanna Pimenta Inada EFFECT OF TECHNOLOGICAL PROCESSES ON PHENOLIC COMPOUNDS CONTENTS OF JABUTICABA (MYRCIARIA JABOTICABA) PEEL AND SEED AND INVESTIGATION OF THEIR ELLAGITANNINS METABOLISM IN HUMANS. Tese de Doutorado apresentada ao Programa de Pós-Graduação em Ciências de Alimentos, Universidade Federal do Rio de Janeiro, como requisito parcial à obtenção do título de Doutor em Ciências de Alimentos Orientadores: Profa. Dra. Mariana Costa Monteiro Prof. Dr. Daniel Perrone Moreira RIO DE JANEIRO 2018 DEDICATION À minha família e às pessoas maravilhosas que apareceram na minha vida. ACKNOWLEDGMENTS Primeiramente, gostaria de agradecer a Deus por ter me dado forças para não desistir e por ter colocado na minha vida “pessoas-anjo”, que me ajudaram e me apoiaram até nos momentos em que eu achava que ia dar tudo errado. Aos meus pais Beth e Miti. Eles não mediram esforços para que eu pudesse receber uma boa educação e para que eu fosse feliz. Logo no início da graduação, a situação financeira ficou bem apertada, mas eles continuaram fazendo de tudo para me ajudar. Foram milhares de favores prestados, marmitas e caronas. Meu pai diz que fez anos de curso de inglês e espanhol, porque passou anos acordando cedo no sábado só para me levar no curso que eu fazia no Fundão. Tinha dia que eu saía do curso morta de fome e quando eu entrava no carro, tinha uma marmita com almoço, com direito até a garrafa de suco. -

Berry Flavonoids and Phenolics: Bioavailability and Evidence of Protective Effects
Downloaded from British Journal of Nutrition (2010), 104, S67–S90 doi:10.1017/S0007114510003958 q The Authors 2010 https://www.cambridge.org/core Berry flavonoids and phenolics: bioavailability and evidence of protective effects Daniele Del Rio1, Gina Borges2 and Alan Crozier2* . IP address: 1Human Nutrition Unit, Department of Public Health, University of Parma, Via Volturno 39, 43100 Parma, Italy 2Plant Products and Human Nutrition Group, School of Medicine, College of Medical, Veterinary and Life Sciences, Graham Kerr 170.106.202.8 Building, University of Glasgow, Glasgow G12 8QQ, UK (Received 25 January 2010 – Accepted 24 February 2010) , on 24 Sep 2021 at 06:36:04 Berries contain vitamin C and are also a rich source of phytochemicals, especially anthocyanins which occur along with other classes of phenolic compounds, including ellagitannins, flavan-3-ols, procyanidins, flavonols and hydroxybenzoate derivatives. This review examines studies with both human subjects and animals on the absorption of these compounds, and their glucuronide, sulphate and methylated metabolites, into the cir- culatory system from the gastrointestinal tract and the evidence for their localisation within the body in organs such as the brain and eyes. The involvement of the colonic microflora in catabolising dietary flavonoids that pass from the small to the large intestine is discussed along with the potential fate and role of the resultant phenolic acids that can be produced in substantial quantities. The in vitro and in vivo bioactivities of these , subject to the Cambridge Core terms of use, available at polyphenol metabolites and catabolites are assessed, and the current evidence for their involvement in the protective effects of dietary polyphenols, within the gastrointestinal tract and other parts of the body to which they are transported by the circulatory system, is reviewed. -

Generating a Qnoael with Confidence Levels from Disparate Literature
University of Kentucky UKnowledge Theses and Dissertations--Pharmacy College of Pharmacy 2018 USING THE QBEST EQUATION TO EVALUATE ELLAGIC ACID SAFETY DATA: GENERATING A QNOAEL WITH CONFIDENCE LEVELS FROM DISPARATE LITERATURE Cynthia Rose Dickerson University of Kentucky, [email protected] Author ORCID Identifier: https://orcid.org/0000-0002-3572-9374 Digital Object Identifier: https://doi.org/10.13023/etd.2018.394 Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Dickerson, Cynthia Rose, "USING THE QBEST EQUATION TO EVALUATE ELLAGIC ACID SAFETY DATA: GENERATING A QNOAEL WITH CONFIDENCE LEVELS FROM DISPARATE LITERATURE" (2018). Theses and Dissertations--Pharmacy. 94. https://uknowledge.uky.edu/pharmacy_etds/94 This Doctoral Dissertation is brought to you for free and open access by the College of Pharmacy at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Pharmacy by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

The Use of Pomegranate (Punica Granatum L.) Phenolic Compounds As Potential Natural Prevention Against Ibds
15 The Use of Pomegranate (Punica granatum L.) Phenolic Compounds as Potential Natural Prevention Against IBDs Sylvie Hollebeeck, Yvan Larondelle, Yves-Jacques Schneider and Alexandrine During Institut des Sciences de la Vie, UCLouvain, Louvain-la-Neuve Belgium 1. Introduction Phenolic compounds (PCs) are plant secondary metabolites that are integral part of the “normal” human diet. The daily intake of PCs depends on the diet but is commonly evaluated at ca. 1g/day for people who eat several fruits and vegetables per day (Scalbert & Williamson, 2000). PCs may be interesting to prevent the development of inflammatory diseases, more particularly in the gastrointestinal tract, where their concentration may reach levels of up to several hundred µM (Scalbert & Williamson, 2000). Many studies have indeed reported on anti-inflammatory properties of different PCs (see (Calixto et al., 2004; Rahman et al., 2006; Romier et al., 2009; Shapiro et al., 2009), for reviews). Pomegranate (Punica granatum L.) belongs to the Punicaceae family, which includes only two species. More than 500 cultivars of Punica granatum exist with specific characteristics such as fruit size, exocarp and aril color, etc. Originating from the Middle East, pomegranate is now widely cultivated throughout the world, and also widely consumed. Pomegranate has been used for centuries in the folk medicine of many cultures. As described in the review of Lansky et al. (2007), the bark and the roots are believed to have anthelmintic and vermifuge properties, the fruit peel has been used as a cure for diarrhea, oral aphthae, and as a powerful astringent, the juice as a blood tonic, and the flowers as a cure for diabetes mellitus. -

Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 7 April 2020 doi:10.20944/preprints202004.0100.v1 Peer-reviewed version available at Appl. Sci. 2020, 10, 3353; doi:10.3390/app10103353 Review Formulation strategies to improve oral bioavailability of ellagic acid Guendalina Zuccari 1,*, Sara Baldassari 1, Giorgia Ailuno 1, Federica Turrini 1, Silvana Alfei 1, and Gabriele Caviglioli 1 1 Department of Pharmacy, Università di Genova, 16147 Genova, Italy * Correspondence: [email protected]; Tel.: +39 010 3352627 Featured Application: An updated description of pursued approaches for efficiently resolving the low bioavailability issue of ellagic acid. Abstract: Ellagic acid, a polyphenolic compound present in fruits and berries, has recently been object of extensive research for its antioxidant activity, which might be useful for the prevention and treatment of cancer, cardiovascular pathologies, and neurodegenerative disorders. Its protective role justifies numerous attempts to include it in functional food preparations and in dietary supplements not only to limit the unpleasant collateral effects of chemotherapy. However, ellagic acid use as chemopreventive agent has been debated because of its poor bioavailability associated to low solubility, limited permeability, first pass effect, and interindividual variability in gut microbial transformations. To overcome these drawbacks, various strategies for oral administration including solid dispersions, micro-nanoparticles, inclusion complexes, self- emulsifying systems, polymorphs have been proposed. Here, we have listed an updated description of pursued micro/nanotechnological approaches focusing on the fabrication processes and the features of the obtained products, as well as on the positive results yielded by in vitro and in vivo studies in comparison to the raw material. -

Ellagitannins in Cancer Chemoprevention and Therapy
toxins Review Ellagitannins in Cancer Chemoprevention and Therapy Tariq Ismail 1, Cinzia Calcabrini 2,3, Anna Rita Diaz 2, Carmela Fimognari 3, Eleonora Turrini 3, Elena Catanzaro 3, Saeed Akhtar 1 and Piero Sestili 2,* 1 Institute of Food Science & Nutrition, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Bosan Road, Multan 60800, Punjab, Pakistan; [email protected] (T.I.); [email protected] (S.A.) 2 Department of Biomolecular Sciences, University of Urbino Carlo Bo, Via I Maggetti 26, 61029 Urbino (PU), Italy; [email protected] 3 Department for Life Quality Studies, Alma Mater Studiorum-University of Bologna, Corso d'Augusto 237, 47921 Rimini (RN), Italy; [email protected] (C.C.); carmela.fi[email protected] (C.F.); [email protected] (E.T.); [email protected] (E.C.) * Correspondence: [email protected]; Tel.: +39-(0)-722-303-414 Academic Editor: Jia-You Fang Received: 31 March 2016; Accepted: 9 May 2016; Published: 13 May 2016 Abstract: It is universally accepted that diets rich in fruit and vegetables lead to reduction in the risk of common forms of cancer and are useful in cancer prevention. Indeed edible vegetables and fruits contain a wide variety of phytochemicals with proven antioxidant, anti-carcinogenic, and chemopreventive activity; moreover, some of these phytochemicals also display direct antiproliferative activity towards tumor cells, with the additional advantage of high tolerability and low toxicity. The most important dietary phytochemicals are isothiocyanates, ellagitannins (ET), polyphenols, indoles, flavonoids, retinoids, tocopherols. Among this very wide panel of compounds, ET represent an important class of phytochemicals which are being increasingly investigated for their chemopreventive and anticancer activities. -

Inhibitory Effect of Tannins from Galls of Carpinus Tschonoskii on the Degranulation of RBL-2H3 Cells
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Tsukuba Repository Inhibitory effect of tannins from galls of Carpinus tschonoskii on the degranulation of RBL-2H3 Cells 著者 Yamada Parida, Ono Takako, Shigemori Hideyuki, Han Junkyu, Isoda Hiroko journal or Cytotechnology publication title volume 64 number 3 page range 349-356 year 2012-05 権利 (C) Springer Science+Business Media B.V. 2012 The original publication is available at www.springerlink.com URL http://hdl.handle.net/2241/117707 doi: 10.1007/s10616-012-9457-y Manuscript Click here to download Manuscript: antiallergic effect of Tannin for CT 20111215 revision for reviewerClick here2.doc to view linked References Parida Yamada・Takako Ono・Hideyuki Shigemori・Junkyu Han・Hiroko Isoda Inhibitory Effect of Tannins from Galls of Carpinus tschonoskii on the Degranulation of RBL-2H3 Cells Parida Yamada・Junkyu Han・Hiroko Isoda (✉) Alliance for Research on North Africa, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan Takako Ono・Hideyuki Shigemori・Junkyu Han・Hiroko Isoda Graduate School of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan Corresponding author Hiroko ISODA, Professor, Ph.D. University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan Tel: +81 29-853-5775, Fax: +81 29-853-5776. E-mail: [email protected] 1 Abstract In this study, the anti-allergy potency of thirteen tannins isolated from the galls on buds of Carpinus tschonoskii (including two tannin derivatives) was investigated. RBL-2H3 (rat basophilic leukemia) cells were incubated with these compounds, and the release of β-hexosaminidase and cytotoxicity were measured. -

Identification of Novel Urolithin Metabolites in Human Feces And
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. Article Cite This: J. Agric. Food Chem. 2019, 67, 11099−11107 pubs.acs.org/JAFC Identification of Novel Urolithin Metabolites in Human Feces and Urine after the Intake of a Pomegranate Extract Rocío García-Villalba, María V. Selma, Juan C. Espín, and Francisco A. Tomas-Barberá ń* Laboratory of Food & Health, Research Group on Quality, Safety, and Bioactivity of Plant Foods, Department of Food Science and Technology, CEBAS-CSIC, P.O. Box 164, Campus de Espinardo, Murcia 30100, Spain *S Supporting Information ABSTRACT: Urolithins are bioactive gut microbiota metabolites of ellagic acid. Here, we have identified four unknown urolithins in human feces after the intake of a pomegranate extract. The new metabolites occurred only in 19% of the subjects. 4,8,9,10-Tetrahydroxy urolithin, (urolithin M6R), was unambiguously identified by 1H NMR, UV, and HRMS. Three metabolites were tentatively identified by the UV, HRMS, and chromatographic behavior, as 4,8,10-trihydroxy (urolithin M7R), 4,8,9-trihydroxy (urolithin CR), and 4,8-dihydroxy (urolithin AR) urolithins. Phase II conjugates of the novel urolithins were detected in urine and confirmed their absorption, circulation, and urinary excretion. The production of the new urolithins was not specific of any of the known urolithin metabotypes A and B. The new metabolites needed a bacterial 3-dehydroxylase activity for their production, and this is a novel feature as all the previously known urolithins maintained the hydroxyl at 3 position.