File No. EC/19/000069 Government of India Ministry of Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Managing Medicolegal Aspects
Published online: 2021-06-08 Medico‑Legal How can we Doctors Protect Ourselves - Managing Medicolegal Aspects Medical practice has undergone a sea change over the last strategies to minimize errors and to bring appropriate few decades. Consumer Protection Act is now applicable to changes to safeguard their clinical practice. Various aspects medical profession also. This has affected the doctor–patient of medicolegal issues such as keeping medical records, relationship adversely and has converted it to one between issuing medical certificates, and discussion about medical service provider and customer. There has been sustained negligence, consent, Consumer Protection Act, various rise in lawsuits as well as violence against hospitals and other relevant acts, and licensing aspects will be covered in doctors. It has become necessary for every practicing upcoming issues of this journal. doctor to stay abreast of the ever‑changing medical laws We are sure our readers will receive this medicolegal section to protect him/herself, his/her family, and medical practice. well to make appropriate changes in their medical practice Unfortunately, medical curriculum in India does not cover to safeguard themselves from medicolegal problems and medicolegal aspects in‑depth, and hence, there is as such practice fearlessly. To ask your doubts/questions, write to dire need to bridge this gap. us on [email protected]. We will try to address the queries Patients are increasingly aware of their rights with easy in due course of time. Happy reading! availability of information on internet and access to medical Sujit Nilegaonkar, Padmaj Kulkarni1 journals. Poor communication skills and increasing medical Departments of Nuclear Medicine and 1Medical Oncology, expenses can create dissatisfaction and lack of trust in Deenanath Mangeshkar Hospital, Pune, Maharashtra, India doctors, which, in turn, can lead to medicolegal complaints. -
STATEMENT “A” Page 1 Status of Influenza a (H1N1) in the State of Maharashtra on 17-06-10
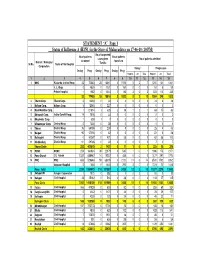
STATEMENT “A” Page 1 Status of Influenza A (H1N1) in the State of Maharashtra on 17-06-10 (10 PM) No.of suspected No.of patients No.of patients cases given No.of patients admitted District / Municipal screened found +ve Sr.No. Name of the Hospital Tamiflu Corporation Onday Progressive Onday Prog Onday ProgOnday Prog Suspect +ve Total Suspect +ve Total 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 1 BMC Kasturba & other Hosp. 32 70683 28 16641 3 1576 5 2 7 1210 131 1341 J. J. Hosp. 0 4830 0 1131 0 160 0 0 0 51 8 59 Private hospital 1 1982 0 1044 2 86 3 0 3 323110 433 33 77495 28 18816 5 1822 8 2 10 1584 249 1833 2 Thane Corp. Thane Corp. 0 12690 0 39 0 4 0 0 0 34 4 38 3 Kalyan Corp. Kalyan Corp. 512065 0 227 0 0 0 0 0 0 0 0 4 Navi Mumbai Corp. 1 121894 1 326 0 16 1 0 1 68 15 83 5 Bhivandi Corp. Indira Gandhi Hosp. 19 7578 0 34 0 0 00 0 20 2 6 Bhayinder Corp. 1 3591 70000 0 00 0 7 Ulhasnagar Corp. Central Hosp 0 7336 0 38 0 0 00 0 40 4 8 Thane District Hosp. 75 68799 0 238 0 8 0 0 0 25 8 33 9 Raigad District Hosp. 90 57096 0 62 0 5 0 0 0 23 5 28 10 Ratnagiri District Hosp. 0 90267 0 421 0 38 0 0 0 62 38 100 11 Sindhudurg District Hosp. -
List of Valued Users
LIST OF VALUED USERS USERS OF BRA - CT ASSISTED STEREOTACTIC FRAME Dr. Charudatta Apte – Neurosurgeon [Chairmen] Prof. S. KALYANARAMAN Dr. V.R. Parikh Sahyadri Hospital & Pune Institute of Neurology Apollo Hospital Enterprise Ltd., Nanavati Hospital Plot no.30 C, Lane no.1, Ali Tower, IV Floor, V i l e p a r l e Prabhat Road, Erandawane 22, Greams Road, P.B. No. 6234 Mumbai - 400 058 Pune – 411 004 ( MAHARASHTRA ) India Madras – 600 006 ( TAMILNADU ) India (MAHARASHTRA) India Ph. : 91 - 020 – 25458370 Ph. : 91 - 044 – 24363551 Ph. : 91-022-26247535 USERS OF BRA-CT ASSISTED STEREOTACTIC FRAME WITH BRK-ARC SYSTEM TARGET ARC CENTERED SOLID ANGLE PRINCIPLE (TASCA) Dr. Dilip Kiyawat Advance Scanning Research Institute Pvt. Ltd. Dr. Ramakrishna Eswaran Jahangir Hospital 11, East High Court Road, Sea Horse Hospitals Ltd. 32, Sassoon Road, Ramdas Peth, (Kanchipura) 6, Royal Road, Trichi – 620 001 Pune – 411 001 ( MAHARASHTRA ) India Nagpur – 440 010 ( MAHARASHTRA ) India Ph. : 91 - 0431 – 2462660, 2462946 Ph. : 91 - 020 – 26122551, 26120801, 26129283® Ph. : 91 - 0712-2529147/8 Mobile – 91 – 98424 – 77688 Dr. Hemang Vasavada Dr. Sanjay Vhora Dr. S, Manoharan Madhuram Hospital Poona Medical Foundation Ganeshpria, 185, Opp. Bhakti Nagar Society, Dhebar Road, Ruby Hall Clinic North Veli Street, Rajlkot – 360 002 ( GUJARAT ) India 40, Sassoon Road, Post Box No.70, Madurai – 625 001 ( TAMILNADU ) Ph. : 91 - 0281–2364088, 2378876, 2376229 Pune – 411 001 ( MAHARASHTRA ) India Ph. : 91 - 0452 – 2342668, 2342278 Mobile – 91 – 98240 41514 Ph. : 91 - 020 – 26123391 (8 Lines Mobile - 91 – 98430 58280 Dr, Sushen Chattopadhyay Dr. Sanjeev Huzurbazar Dr. Bhargav Trivedi Health Point, 21/1, Neurosurgery Centre Riddhi Nursing Home Prannath Pandit Street, Sushrut Truma Care Centre 1, Sun Silver Apts, Productivity Road, Kolkata – 700 025 ( WEST BENGAL) India 344, Navi Peth, Acharya Complex, Near Akota Garden, Akota, Baroda Ph. -

HNS - Head and Neck Surgery, Larynx and Others
Eur Arch Otorhinolaryngol (2007) (Suppl 1) 264:S5–S151 DOI 10.1007/s00405-007-0344-7 HNS - Head and Neck Surgery, Larynx and Others INSTRUCTIONAL COURSES indication for EPT in patients with squamous cell cancer of the upper aerodigestive tract. HIC 1 Thyroid surgery and dealing with complications HIC 3 Jan Betka Digital volume tomography in oto-rhino-laryngology Department of Otorhinolaryngology and Head and Neck Surgery, 1st Faculty of Medicine, Charles University, Faculty Hospital Carsten Dalchow Motol, V U´ valu 84, 150 06 Prague 5, Czech Republic Park-Klinik Weissensee, Scho¨ nstr. 80, 13089 Berlin, Germany The course provides overview of technique of thyroid surgery The digital volume tomography (DVT) is an extension of pano- including both standard and up-to-date modern methods includ- ramic tomography. With this diagnostic technique, characterized ing not-cold instruments (harmonic knife), miniinvasive methods, by high resolution, minimal section thickness of 0.125 mm, and endoscopic thyroid surgery. The extent of surgery (total thyroid- three-dimensional (3D) display, small pathological processes can ectomy, hemithyroidectomy) is discussed. Various procedures for be well visualized. identification of the recurrent nerve (including nerve monitoring) The digital volume tomograph Accu-I-tomo (Morita, Kyoto, and parathyroid glands are shown. The question how to drain (if Japan) was routinely used to examine patients with a history of a any) the wound is gone over. Finally special focus is aimed at disease in the field of oto-rhino-lanyngology. A 3D dataset of a dealing with complications—recurrent nerve palsy (unilateral, cylinder was obtained in one 360° rotation with 80 kV and 8 mA bilateral), parathyroid gland injury. -

Dr. Arvind Bhave
Dr. Arvind Bhave Dr. Arvind Bhave is a specialized spine surgeon trained in various prestigious spinal surgery units in the world. Obtained training in Neuro-spinal and spinal surgery at the following centers : Tel Aviv University, Israel. Spinal Injury Centre, Fukuoka, Japan. Wooridul Spine Hospital, Korea, for Endoscopic Surgery. EDUCATIONAL QUALIFICATION Bachelor Of Medicine & Bachelor Of Surgery (M.B.B.S.) – B.J.Medical College, University Of Pune, in year 1985. Master Of Surgery (Orthopaedic Surgery) (M.S.) – University Of Pune, India in year 1989. International Postgraduate Training Course In Medicine – Orthopaedic Surgery – Tel Aviv University Telaviv (Israel) – Spine Surgery in year 1998. Fellow Spinal Injuries Centre in Fukuoka, Japan in year 2001. Fellowship – Minimally Invasive Spine Surgery – Wooridul Spine Hospital, Seoul, Korea in year 2003 LANGUAGES English, Hindi, Marathi, Sanskrit, German, Hebrew, Japanese. PROFESSIONAL EXPERIENCE (Journey) 1985-86 -Internship Training In Clinical Medicine & Surgery 1987-89 - Resident Doctor In Orthopaedic Surgery. 1989-91 -Orthopaedic Surgeon – Esis Hospital (Govt.of Maharashtra). Since1991-Consulting Pine /Orthopedic Surgeon Bhave Hospital 1991-98 -Lecturer In Orthopedics, Dept. Of Orthopaedics, Bharti Vidyapeeth Medical College Pune. Associate Professor Of Orthopaedics, Dept. Of Orthopaedics Bharti Vidyapeeth Medical College. 1998-2002 -Associate Professor Of Orthopedics Sancheti Institute Of Orthopedics & Rehabilitation – 4 Years. 2006 -Professor Of Orthopedics Bharti Vidyapeeth Medical College. Selected By Maharashtra Public Service Commission[MPSE]. Visiting Orthopedic Surgeon For International Postgraduate Training Programme In Orthopedic Surgery-Tel-Aviv University, Israel. Achievements PRESENT POSITION PAPERS & PUBLICATIONS AWARDS RECEIVED FACULTY POSITIONS BOOKS AUTHORED PRESENT POSITION Honorary Spine consultant Deenanath Mangeshkar Hospital, Pune. Orthopaedic spine surgeon & incharge Bhave hospital, Pune. -

Bollywood Sounds
Bollywood Sounds Bollywood Sounds The Cosmopolitan Mediations of Hindi Film Song Jayson Beaster-Jones 1 1 Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford New York Auckland Cape Town Dar es Salaam Hong Kong Karachi Kuala Lumpur Madrid Melbourne Mexico City Nairobi New Delhi Shanghai Taipei Toronto With offices in Argentina Austria Brazil Chile Czech Republic France Greece Guatemala Hungary Italy Japan Poland Portugal Singapore South Korea Switzerland Thailand Turkey Ukraine Vietnam Oxford is a registered trademark of Oxford University Press in the UK and certain other countries. Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016 © Oxford University Press 2015 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by license, or under terms agreed with the appropriate reproduction rights organization. Inquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above. You must not circulate this work in any other form and you must impose this same condition on any acquirer. Library of Congress Cataloging-in-Publication Data Beaster-Jones, Jayson. Bollywood sounds : the cosmopolitan mediations of Hindi film song / Jayson Beaster-Jones. pages cm Includes bibliographical references and index. ISBN 978–0–19–986254–2 (pbk. -

A One-Stop State of the Art, Non-Profit Healthcare Facility in Pune, India
HUMANITARIAN NEWS A one-stop state of the art, non-profit healthcare facility in Pune, India In 2001, a non-profit healthcare facility was founded in Pune India, providing low and middle income group patients with treatment, not only at a minimum cost, but of a quality which bears the stamp of approval by the Royal College of Surgeons of England. Mr Vasant Oswal, an integral figure in the evolution of the hospital, speaks to other key members involved in its development. eenanath Mangeshkar Hospital (DMH) is a 1000-bed hospital in Pune, India, with a 25,000 square Dfeet dedicated postgraduate study centre which has established several surgical training courses. In recognition of their high educational quality, Royal College of Surgeons (RCS) has awarded the facility accreditation as ’a Centre of Surgical Education and Training’ making it the first centre to achieve this status in India. The RCS has also approved the first overseas RCS Senior Clinical Fellowship at the laryngology department of the hospital for a period of three years. Inauguration of Vasant and Nirmal Oswal Centre for Postgraduate Training. From right to left: Prakash Jawadekar, Union HRD From left to right, Dr Sachin Gandhi, Mr Paul O’Flynn, Mr Vasant Oswal, Minister, Government of India presenting bouquet Mr Prakash Javadekar, Dr Jay Kelkar. to Nirmal Oswal. Dr Dhananjay Kelkar, (52 neonates and 12 paediatric) caters for Medical Trust to establish, administer and Medical Director, critical care. Robotics and an extension of the promote the healthcare facility, with the Deenanath Mangeshkar stem cell transplant facility is in the pipeline. -

The and Me Ahatma
The Prabha Khaitan Foundation Chronicle September 2020 I Issue 18 The idea of Mohandas Karamchand Gandhi is eternal, his message universal. In this issue, 14 writers from all walks of life share their experience of encountering the Mahatma's legacy in their everyday lives The Mahatma and Me Pg 4-28 TÊTE-À-TÊTE THE IDENTITY MUSICAL WITH TULLY OF AN INDIAN MUSINGS 40 48 44, 52 INSIDE 2 INSIDE IN PURSUIT OF MANISHA JAIN Communications & Branding Chief, SUCCESS Prabha Khaitan Foundation 30 DAUGHTERS, THE Musings about the DRIVERS OF CHANGE 31 Mahatma and More DIFFERENT STROKES nprecedented" is a word we've all heard many times in the past few months as the pandemic wreaked havoc OF LITERATURE through the world. However, the spirit of the festive 32 Useason bears the fragrance of happiness and hope for better times. I take this opportunity to urge everyone to celebrate THE STORY OF responsibly, in a scaled-down fashion that curbs the spread of the coronavirus, and helps reduce the risk of infecting the ones SARDAR PATEL you love. 36 A few months back, I had read about an organisation in South India that appealed to people to undertake voluntary CELEBRATING HINDI fasting for a day from 6 am to 6 pm as atonement towards 39 the migrants and the marginalised. It was clearly a Gandhian approach to bring about social reform. According to Gandhi, DECODING DEFENCE "What the eyes are for the outer worlds, fasts are for the inner". The philosophies and teachings of Mahatma Gandhi and his 42 vision of India have taken on a special relevance in today's circumstances, whether it is his adherence to truth or non- THE PULL OF violence. -

Classical Singers
1. RAVI SHANKAR Ravi Shankar (7 April 1920 – 11 December 2012), often referred to by the title Pandit, was an Indian musician and composer who played thesitar, a plucked string instrument. He has been described as the best-known contemporary Indian musician. He spent his youth touring Europe and India with the dance group of his brother Uday Shankar. He gave up dancing in 1938 to study sitar playing under court musician Allauddin Khan. After finishing his studies in 1944, Shankar worked as a composer, creating the music for the Apu Trilogy by Satyajit Ray, and was music director of All India Radio, New Delhi, from 1949 to 1956. In 1956, he began to tour Europe and the Americas playing Indian classical music and increased its popularity there in the 1960s through teaching, performance, and his association with violinist Yehudi Menuhin and rock artist George Harrison of the Beatles. Shankar engaged Western music by writing concerti for sitar and orchestra and toured the world in the 1970s and 1980s. From 1986 to 1992 he served as a nominated member of Rajya Sabha, the upper chamber of the Parliament of India. Shankar was awarded India's highest civilian honour, the Bharat Ratna, in 1999, and received three Grammy Awards. He continued to perform in the 2000s, sometimes with his younger daughter, Anoushka. Career Shankar developed a style distinct from that of his contemporaries and incorporated influences from rhythm practices of Carnatic music. His performances begin with solo alap, jor, and jhala (introduction and performances with pulse and rapid pulse) influenced by the slow and serious dhrupad genre, followed by a section with tabla accompaniment featuring compositions associated with the prevalent khyal style. -

Seleted Hospital List
Sr. District Location Name of the Provider Address Address I Address II Ph. No. No. 1 Ahmednagar Ahmednagar Deshpande Hospital, Ahmednagar Patwardhan Chowk, Ahmednagar, Ahmednagar-414 001 0241-2341012 2 Ahmednagar Ahmednagar Deepak Hospital, Ahmednagar Savedi Road, Ahmednagar, Ahmednagar-414 001 0241-2324888 Noble Hospital & Research Center, 3 Ahmednagar Ahmednagar Premdan Chowk, Manmad Road, Savedi Road, Ahmednagar-414003 0241-24212909 Ahmednagar Anand Rushiji Hospital, 4 Ahmednagar Ahmednagar Plot No-124, Anand Rishiji Marg, Ahmednagar-414001 0241-2320474 Ahmednagar Sai Multi Specility Hospital & Near Railway over Bridge, 5 Ahmednagar Shrirampur Newasa Road, Shrirmapur -413709 02422-223760 Research Centre, Ahmednagar Dist. Ahmednagar 6 Ahmednagar Sangamner Ithape Hospital, Sangamner Dist.-Ahmednagar Sangamner-422605 02425-225014 Dr. Ashok Ithape 7 Akola Akola Orbit Children Hospital, Akola Near Krishna Mandir, Ratanlal Plot, Akola-444005 0724-2456800 8 Akola Akola Gajanan Hospital, Akola Akola Akola-444005 0724-2450477 Dr. Mapari Hitech Critical Care & Bonde 9 Amravati Amravati 48 - 3/4, Dastur Nagar Road, Rajapeth, Amravati-444 606 0721-2676416 Hospital, Amravati 10 Amravati Amravati Suyash Hospital, Amravati Near Bus Stand, Rukmini Nagar, Amravati-444606 9370164080 Camp Area, Near Hotel Amravati Amravati Daya Sagar Hospital, Amravati Amravati-444606 8805346997 11 Mahefil, Amravati Amravati Cancer Foundation, Kaval Nagar square, shree 12 Amravati Amravati Shankar Nagar, Amravati-444606 9823097573 Amravati complex, Kamalnayan Bajaj Hospital, -

Deenanath Mangeshkar Hospital
+91-8048372326 Deenanath Mangeshkar Hospital https://www.indiamart.com/deenanath-mangeshkar-hospital/ At DeSoto Memorial Hospital, we understand that healthcare is personal and we only get one chance to make a good first impression. Our goal is to provide every member of our community with the highest level of healthcare services and exceed your ... About Us At DeSoto Memorial Hospital, we understand that healthcare is personal and we only get one chance to make a good first impression. Our goal is to provide every member of our community with the highest level of healthcare services and exceed your expectations. We offer the latest in medical technology to our community and are continuously recruiting the most qualified physicians to provide services. Since the Board of Directors have entrusted the day to day operation of the Hospital to me, my only direction is to make decisions based on one simple premise: "If it is good for the Hospital, it is the right thing to do." I take every success and every failure personally, and I want to know if we fail to meet your expectations. DeSoto Memorial Hospital can and should be your healthcare provider and your primary source of healthcare in our community. Please enjoy our website and use it to become familiar with all of our products and services. If we can answer any other questions please contact us directly. Remember, we are here to serve you, our community. Come in and see why DeSoto Memorial Hospital will exceed your expectations. For more information, please visit https://www.indiamart.com/deenanath-mangeshkar-hospital/aboutus.html F a c t s h e e t Nature of Business :Service Provider CONTACT US Deenanath Mangeshkar Hospital Contact Person: Meenal Nene Erandawane, Near Mhatre Bridge Pune - 411004, Maharashtra, India +91-8048372326 https://www.indiamart.com/deenanath-mangeshkar-hospital/. -

Dr. Ashish Babhulkar
3,Anant,Income Tax Lane T: 0 2 0 2 5 4 5 0 4 0 4 Off Karve Road M: + 9 1 9 8 2 2 2 1 8 5 5 0 Pune 411016 E: [email protected] I N D I A W: w w w . j o i n t a n d b o n e s . c o m Dr. Ashish Babhulkar D.Orth.,DNB(Orth.).,MCH.(Orth.)(Liverpool,UK),FRCS(Tr.&Orth.) Profession Shoulder Specialist & Joint Replacement Surgeon Qualification · MBBS Jan 1989 · D.Orth Jan 1994 · DNB (Orth.) June 1995 · MCh.Orth. Dec. 1998 · FRCS(Tr. & Orth.) May 2001 Attachments · HEAD Dept of Shoulder & Sports Injuries · Deenanath Mangeshkar Hospital, Pune Gold Medal – Paper on Arthroscopic Adhesiolysis for stiff Prize shoulder, MOACON Goa 2005 Gold Medal – Reconstruction of AC joint using Gracilis graft - IOACON Bangalore Dec 2008 Gold Medal – MOACON, Nasik – Results of Arthroscopic Suprascapualar nerve decompression 29-31 Oct 2010 Gold Medal – Shoulder Elbow Con 2015 – Delhi – Bony Morphology of Shoulder- Indian Cadaver study of 66 Shoulders. Best research paper award - Deenanath Mangeshkar Hospital for the year 2017 Membership • Indian Orthopaedic Association • Indian Arthroscopy Society • Asian Federation of Sports Medicine • Maharashtra Orthopaedic Association • Poona Orthopaedic Society • Bombay Orthopaedic Society • Indian Association of Sports Medicine • Indian Federation of Sports Medicine • Bone & Joint Decade –NAN • Indian Menopause Society • Shoulder & Elbow Society, India • SICOT • Hon. Member SESA – Shoulder & Elbow society, Australia • Founder President Shoulder Society of India • Treasurer – Indian Menopause Society, Pune chapter Nov 2013 onwards • Member Shoulder & Elbow Committee – SICOT • Member Asian Shoulder & Elbow Group • Reviewer for International Journal of Shoulder Surgery • British Journal of Shoulder & Elbow Surgery • Indian Journal of Orthopedics • Journal for clinical Orthopedics, Bombay Orth.