Low Nuclearity Gold Clusters, Structure and Bonding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gold Surfaces and Nanoparticles Are Protected by Au(0)
Gold surfaces and nanoparticles are protected PNAS PLUS by Au(0)–thiyl species and are destroyed when Au(I)–thiolates form Jeffrey R. Reimersa,b,1, Michael J. Fordb, Arnab Halderc, Jens Ulstrupc, and Noel S. Hushd,e,1 aInternational Centre for Quantum and Molecular Structures, College of Sciences, Shanghai University, Shanghai 200444, China; bSchool of Mathematical and Physical Sciences, The University of Technology Sydney, Sydney NSW 2007, Australia; cDepartment of Chemistry, Technical University of Denmark, Kongens Lyngby 2800, Denmark; dSchool of Chemistry F11, The University of Sydney, Sydney NSW 2006, Australia; and eSchool of Molecular Bioscience, The University of Sydney, Sydney NSW 2006, Australia Contributed by Noel S. Hush, January 15, 2016 (sent for review March 2, 2015; reviewed by William Goddard, Mark Gordon, and David J. Schiffrin) The synthetic chemistry and spectroscopy of sulfur-protected gold interactions between charged tail groups can also inhibit adatom surfaces and nanoparticles is analyzed, indicating that the elec- formation (17). SAMs involving adatoms have poor long- tronic structure of the interface is Au(0)–thiyl, with Au(I)–thiolates range order owing to the surface pitting that is required to identified as high-energy excited surface states. Density-functional deliver gold adatoms, while directly bound motifs lead to regular theory indicates that it is the noble character of gold and nano- surfaces (18). particle surfaces that destabilizes Au(I)–thiolates. Bonding results There is clearly a delicate balance between the forces that from large van der Waals forces, influenced by covalent bonding direct these different interface structures, a balance that can only induced through s–d hybridization and charge polarization effects be understood through knowledge of the electronic structures of that perturbatively mix in some Au(I)–thiolate character. -

Investigations Into Ligand Substitutions of Rhenium
INVESTIGATIONS INTO LIGAND SUBSTITUTIONS OF RHENIUM AND MOLYBDENUM d4 HEXANUCLEAR CLUSTERS AND THE SYNTHESIS AND CHARACTERIZATION OF AURATED PYRENE AND THIOPHENE DERIVATIVES By MIYA ALETHEA PEAY Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Thesis Adviser: Dr. Thomas Gray Department of Chemistry CASE WESTERN RESERVE UNIVERSITY August, 2011 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of _____Miya Peay________________________________________ candidate for the ______Doctorate of Philosophy _____degree *. (signed)_____John Protasiewicz____________________________ (chair of the committee) ___________ Irene Lee ____________________________ ___________ John Stuehr __________________________ __ Anthony Berdis __ _____________________ ___ Thomas Gray__________________________ ________________________________________________ (date) ___August 2011__________ *We also certify that written approval has been obtained for any proprietary material contained therein. Dedicated To: God the Father, the Son, and the Holy Spirit, the One who strengthens me and keeps me, To my family and friends for their undying love and support throughout the many years. I could not have done any of this without any of you! Finally to my unborn baby girl, Mommy has loved you from the day I found out about you. You mean more to me than I could have ever imagined and I can’t wait to show this to you in hopes that one day you’ll see it as a means to push yourself to greater -

Relativistic Effects in Chemistry: More Common Than You Thought
PC63CH03-Pyykko ARI 27 February 2012 9:31 Relativistic Effects in Chemistry: More Common Than You Thought Pekka Pyykko¨ Department of Chemistry, University of Helsinki, FI-00014 Helsinki, Finland; email: pekka.pyykko@helsinki.fi Annu. Rev. Phys. Chem. 2012.63:45-64. Downloaded from www.annualreviews.org Annu. Rev. Phys. Chem. 2012. 63:45–64 Keywords Access provided by WIB6049 - University of Freiburg on 07/13/18. For personal use only. First published online as a Review in Advance on Dirac equation, heavy-element chemistry, gold, lead-acid battery January 30, 2012 The Annual Review of Physical Chemistry is online at Abstract physchem.annualreviews.org Relativistic effects can strongly influence the chemical and physical proper- This article’s doi: ties of heavy elements and their compounds. This influence has been noted 10.1146/annurev-physchem-032511-143755 in inorganic chemistry textbooks for a couple of decades. This review pro- Copyright c 2012 by Annual Reviews. vides both traditional and new examples of these effects, including the special All rights reserved properties of gold, lead-acid and mercury batteries, the shapes of gold and 0066-426X/12/0505-0045$20.00 thallium clusters, heavy-atom shifts in NMR, topological insulators, and certain specific heats. 45 PC63CH03-Pyykko ARI 27 February 2012 9:31 1. INTRODUCTION Relativistic effects are important for fast-moving particles. Because the average speeds of valence electrons are low, it was originally thought [in fact by Dirac (1) himself ] that relativity then was unimportant. It has now been known for a while that relativistic effects can strongly influence many chemical properties of the heavier elements (2–5). -

Intramolecular D10–D10 Interactions in Heterometallic Clusters of the Transition Metals Sabrina Sculfort, Pierre Braunstein
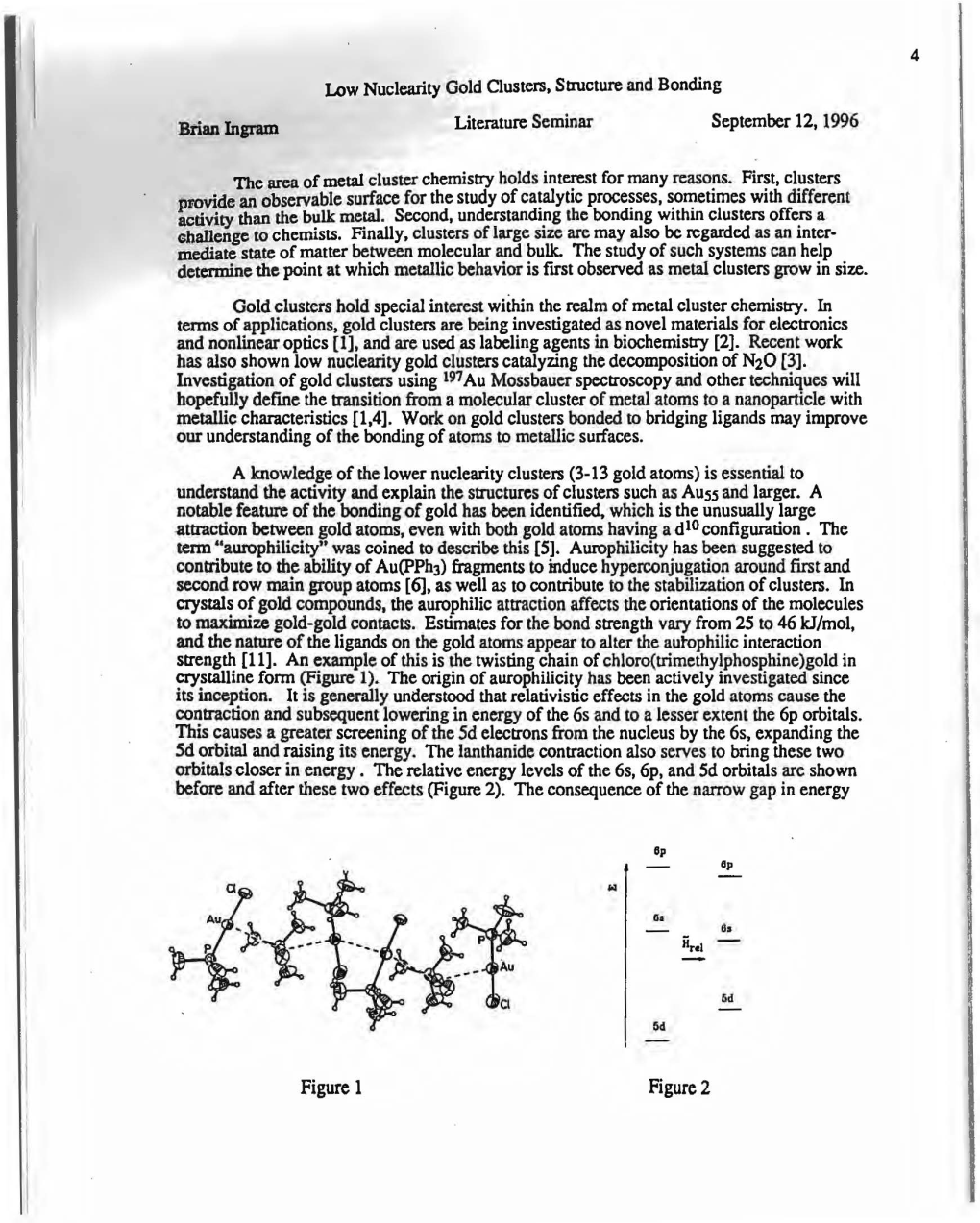
Intramolecular d10–d10 interactions in heterometallic clusters of the transition metals Sabrina Sculfort, Pierre Braunstein To cite this version: Sabrina Sculfort, Pierre Braunstein. Intramolecular d10–d10 interactions in heterometallic clusters of the transition metals. Chemical Society Reviews, Royal Society of Chemistry, 2011, 40 (5), pp.2741- 2760. 10.1039/c0cs00102c. hal-01872459 HAL Id: hal-01872459 https://hal.archives-ouvertes.fr/hal-01872459 Submitted on 20 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Revised CS-CRV-09-2010-000102 Intramolecular d10-d10 interactions in Heterometallic Clusters of the Transition Metals † Sabrina Sculfort and Pierre Braunstein* Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal - CS 90032, F-67081 Strasbourg Cedex, France. E-mail: [email protected] Fax: +33 368 851 322; Tel: +33 368 851 308. † In memoriam Dr. Marie-Madeleine Rohmer 2 For the Table of Contents This review deals with the synthesis and structures of heterometallic transition metal clusters displaying intramolecular, metallophilic d10-d10 interactions. 3 Abstract Weak attractive interactions between closed shell metal ions have been increasingly studied in the last few years and are generally designated as metallophilic interactions. -

Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers
polymers Review Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers David Ulkoski and Carmen Scholz * Department of Chemistry, University of Alabama, 301 Sparkman Dr., Huntsville, AL 35899, USA; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +1-256-824-6188 Received: 7 September 2017; Accepted: 7 October 2017; Published: 11 October 2017 Abstract: The redox capacity, as well as the aurophilicity of the terminal thiol side groups, in poly(Cysteine) lend a unique characteristic to this poly(amino acid) or polypeptide. There are two major application fields for this polymer: (i) biomedical applications in drug delivery and surface modification of biomedical devices and (ii) as coating for electrodes to enhance their electrochemical sensitivity. The intended application determines the synthetic route for p(Cysteine). Polymers to be used in biomedical applications are typically polymerized from the cysteine N-carboxyanhydride by a ring-opening polymerization, where the thiol group needs to be protected during the polymerization. Advances in this methodology have led to conditions under which the polymerization progresses as living polymerization, which allows for a strict control of the molecular architecture, molecular weight and polydispersity and the formation of block copolymers, which eventually could display polyphilic properties. Poly(Cysteine) used as electrode coating is typically polymerized onto the electrode by cyclic voltammetry, which actually produces a continuous, pinhole-free film on the electrode via the formation of covalent bonds between the amino group of Cysteine and the carbon of the electrode. This resulting coating is chemically very different from the well-defined poly(Cysteine) obtained by ring-opening polymerizations. -

Synthesis and Structure of N-Heterocyclic Carbene Gold Sulfide Complexes
SYNTHESIS AND STRUCTURE OF N-HETEROCYCLIC CARBENE GOLD SULFIDE COMPLEXES A Dissertation Presented to The Academic Faculty by Christopher Masanao Sato In Partial Fulfillment of the Requirements for the Degree Master of Science in the School of Chemistry and Biochemistry Georgia Institute of Technology May 2018 COPYRIGHT © 2018 BY CHRISTOPHER MASANAO SATO SYNTHESIS AND STRUCTURE OF N-HETEROCYCLIC CARBENE GOLD SULFIDE COMPLEXES Approved by: Dr. Joseph P. Sadighi, Advisor School of Chemistry and Biochemistry Georgia Institute of Technology Dr. Jake D. Soper School of Chemistry and Biochemistry Georgia Institute of Technology Dr. Henry S. La Pierre School of Chemistry and Biochemistry Georgia Institute of Technology Date Approved: January 12, 2018 TABLE OF CONTENTS LIST OF TABLES v LIST OF FIGURES vi LIST OF SYMBOLS AND ABBREVIATIONS viii LIST OF SCHEMES iv SUMMARY v CHAPTER 1. Introduction 1 1.1 Aurophilicity 1 + 1.2 Isolobal Analogy of Gold (I) and H 2 1.3 Gold Oxo Complexes 3 1.4 References 5 CHAPTER 2. Synthesis of N-Heterocyclic Carbene Trigold Sulfide Complexes 8 2.1 Background 8 2.2 Results and Discussion 10 2.2.1 Synthesis and Structural Characterization of {[(ICy)Au]3(μ3-S)}X and {[(IMes)Au]3(μ3-S)}BF4 Salts 10 2.2.2 X-ray Crystal Structure of {[(ICy)Au]3(μ3-S)}OTs 11 2.2.3 X-ray Crystal Structure of {[(IMes)Au]3(μ3-S)}Cl 12 2.3 Conclusion 14 2.4 Experimental 14 2.4.1 General Considerations 14 2.4.2 Synthetic Procedures 15 iii 2.4.3 X-ray Diffraction Data 22 2.5 References 23 CHAPTER 3. -

AND SILVER(I) THIOLATE COMPLEXES of MEDICINAL INTEREST: a REVIEW and RECENT RESULTS Helen E
STRUCTURES OF GOLD(I) AND SILVER(I) THIOLATE COMPLEXES OF MEDICINAL INTEREST: A REVIEW AND RECENT RESULTS Helen E. Howard-Lock Laboratories for Inorganic Medicine, McMaster University, Hamilton, ON, Canada L8S 4M1 Abstract: We review the crystal structures, electrospray ionization mass spectra (ESI-MS) data and chiral HPLC data for the racemic and optically pure mononuclear AuL2 complexes, and for racemic [AuLI]n and optically pure [AgLII]n polymers (LI thiomalate, LII 13-penicillamine). We postulate an equilibrium between polymeric, mononuclear and free ligand species for [AuLII]n (gold sodium thiomalate or GST). The ESI-MS results clearly show a tetrameric principal species in the 1:1 gold polymers, [AuLI]n. For the 1:1 silver: 13- penicillamine complex, [AgLII]n, a non-molecular crystal of double helical structure, the ESI-MS results show multi-ligand-silver species, including tetramers, pentamers and hexamers. Other, relevant gold, silver and copper complexes are compared. Aurophilicity Chemists should stop expressing surprise when short Au...Au distances appear in structures of gold comploexes. In a review of about 700 structures containing Au-S bonds, Au-Au contacts range from 2.50 to 4.00 A. It is difficult to define a Au-Au contact as bonded or non-bonded by distance alone, but gold-gold interactions occur within narrow distance ranges, and the orientation of gold atoms in crystals are characterized by specific anogular geometries. Arbitrarily the distances 2.55-3.10 A have been assigned to Au-Au bonds; 2.90-3.30 A to Au...Au non-bonded intramolecular interactions; and 2.80-3.40 ,, to intermolecular contacts between molecular species with a single 9old atom. -

Advances in Gold-Carbon Bond Formation: Mono-, Di-, and Triaurated Organometallics
ADVANCES IN GOLD-CARBON BOND FORMATION: MONO-, DI-, AND TRIAURATED ORGANOMETALLICS By JAMES E. HECKLER Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Thesis Advisor: Dr. Thomas G. Gray Department of Chemistry CASE WESTERN RESERVE UNIVERSITY January 2016 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of ____________________________________________________James E. Heckler candidate for the _____________________________Doctor of Philosophy degree*. Carlos E. Crespo-Hernandez (Signed) __________________________________ (chair of the committee) Anthony J. Pearson __________________________________ __________________________________Genevieve Sauve __________________________________Horst von Recum __________________________________Thomas G. Gray (date) ____________________27 July 2015 * We also certify that written approval has been obtained for any proprietary material contained therein. Dedication To my family and best friends i Table of Contents List of Tables …………………………………………………………………………………….iii List of Figures ……………..………………………………………………………………….......v List of Schemes and Charts..…………………………………………………………………..…..x Acknowledgements ……………………………………………………………………….……..xii List of Symbols and Abbreviations ……………………………………………………….....…xiii Abstract ……………………………………………………………………………………..…..xxi Chapter 1. General Introduction ………………….......................................................1 1.1 Fundamental gold chemistry………………………………………………1 1.1.1 Relativistic effects -

The Chemistry of Gold M
j1 1 The Chemistry of Gold M. Concepción Gimeno 1.1 Introduction 1.1.1 History Gold was discovered as shining yellow nuggets and is undoubtedly the first metal known to early civilizations. The symbol derives from the Latin word aurum, which is related to the goddess of dawn, Aurora. Early civilizations equated gold with gods and rulers, and gold was sought in their name and dedicated to their glorification. Humans almost intuitively attribute a high value to gold, associating it with power, beauty, and the cultural elite. And since gold is widely distributed all over the globe, it has been perceived in the same way throughout ancient and modern civilizations everywhere. Archeological digs suggest gold was first used in the Middle East where the first known civilizations developed. Experts in the study of fossils have observed that pieces of natural gold were found in Spanish caves used by Paleolithic Man in about 40 000 BC. The oldest pieces of gold jewellery were discovered in the tombs of Queen Zer of Egypt and Queen Pu-abi of Ur in Sumeria and date from the third millennium BC. Most Egyptian tombs were raided over the centuries, but the tomb of Tutankhamun was discovered undisturbed by modern archeologists. The largest collection of gold and jewellery in the world included a gold coffin whose quality showed the advanced state of Egyptian craftsmanship and goldworking (second millennium BC). The Persian Empire, in what is now Iran, made frequent use of gold in artwork as part of the religion of Zoroastrianism. Persian goldwork is most famous for its animal art, which was modified after the Arabs conquered the area in the seventh century AD. -

Dinuclear Metal-Mediated Homo Base Pairs with Metallophilic
www.nature.com/scientificreports OPEN Dinuclear Metal-Mediated Homo Base Pairs with Metallophilic Interactions: Theoretical Studies of Received: 24 March 2017 2+ Accepted: 9 October 2017 G2M2 (M = Cu, Ag, and Au) Ions Published: xx xx xxxx Guo-Jin Cao Dinuclear metal-mediated homo base pairs are interesting clusters with highly symmetric structures 2+ and significant stabilities. The geometric and electronic structures of 2G M2 (G = Guanine, M = Cu, Ag or 2+ Au) cluster ions were studied with quantum chemical calculations. The lowest-energy isomers of G2M2 cluster ions have C2h symmetries with an approximately antiparallel alignment of two sets of N-M∙∙∙O groups being formed in the planar structures. The M-M distances are shorter than the sum of van der Waals radii of corresponding two homo coinage metal atoms, showing that metallophilic interactions significantly exist in these complexes. They have the large HOMO−LUMO gaps of about 5.80 eV at the DFT level and the bond dissociation energies of more than 5.60 eV at the DFT/B3LYP level, indicating that these cluster dications are highly stable. The second lowest-energy isomers stabilized by an approximately parallel alignment of one set of O-M-O group and one set of N-M-N group are found to 2+ be close to the lowest-energy isomers in energy. The barrier between the two isomers of G2M2 cluster ions is significantly large, also showing that these lowest-energy isomers are very stable. Metal ion-base pair complexes have attracted tremendous attention because of their importance in the devel- opment of nanotechnology and biotechnology1–8. -

1 Inorg. Chem. 2009, 48, 3866-3874
1 Inorg. Chem. 2009, 48, 3866-3874 Multimetallic arrays: Bi-, tri-, tetra- and hexametallic complexes based on gold(I) and gold(III) and the surface functionalisation of gold nanoparticles with transition metals. Edward R. Knight,a Nina H. Leunga Amber L. Thompson,a G. Hogarthb and James D. E. T. Wilton-Elya,c§* a) Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. b) Department of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK. c) Department of Chemistry, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: [email protected] § Current address: Imperial College London. Keywords: gold, dithiocarbamate, multimetallic, aurophilicity, nanoparticle 2 Abstract Reaction of [AuCl(PPh3)] with the zwitterion S2CNC4H8NH2 yields [(Ph3P)Au(S2CNC4H8NH2)]BF4. Treatment of this species with NEt3 and CS2 followed by [AuCl(PPh3)] leads to [{(Ph3P)Au}2(S2CNC4H8NCS2)], which can also be obtained directly from [AuCl(PPh3)] and KS2CNC4H8NCS2K. A heterobimetallic variant, + [(dppm)2Ru(S2CNC4H8NCS2)Au(PPh3)] , can be prepared by the sequential reaction of 2+ [(dppm)2Ru(S2CNC4H8NH2)] with NEt3 and CS2 followed by [AuCl(PPh3)]. Reaction of the same ruthenium precursor with [(dppm)(AuCl)2] under similar conditions yields the trimetallic complex 2+ [(dppm)2Ru(S2CNC4H8NCS2)Au2(dppm)] . Attempts to prepare the compound 2+ [(dppm)Au2(S2CNC4H8NH2)] from [(dppm)(AuCl)2] led to isolation of the known complex 2+ [{(dppm)Au2}2(S2CNC4H8NCS2)] via a symmetrization pathway. 2+ [{(dppf)Au2}2(S2CNC4H8NCS2)] was successfully prepared from [(dppf)(AuCl)2] and crystallographically characterized. In addition, a gold(III) trimetallic compound, 3+ [{(dppm)2Ru(S2CNC4H8NCS2)}2Au] , and a tetrametallic gold(I) species, 2+ [{(dppm)2Ru(S2CNC4H8NCS2)Au}2] , were also synthesized. -
![[{(H3C)3NB(H)2NC}2Au][Aui2]: a Linear Chain Polymer of Gold(I) Iodide with an Unusual Isocyanoborane Ligand Showing Aurophilic Behaviour](https://docslib.b-cdn.net/cover/2527/h3c-3nb-h-2nc-2au-aui2-a-linear-chain-polymer-of-gold-i-iodide-with-an-unusual-isocyanoborane-ligand-showing-aurophilic-behaviour-4322527.webp)
[{(H3C)3NB(H)2NC}2Au][Aui2]: a Linear Chain Polymer of Gold(I) Iodide with an Unusual Isocyanoborane Ligand Showing Aurophilic Behaviour
metal-organic papers Acta Crystallographica Section E [{(H C) NB(H) NC} Au][AuI ]: a linear chain Structure Reports 3 3 2 2 2 Online polymer of gold(I) iodide with an unusual ISSN 1600-5368 isocyanoborane ligand showing aurophilic behaviour William C. Kaska,a Hermann A. Treatment of the (isocyanoborane)gold(I) chloride adduct Received 29 March 2004 b c Accepted 2 April 2004 Mayer, Mark R. J. Elsegood, [LAuCl] [L =(H3C)3NB(H)2NC] with KI at room tempera- Online 17 April 2004 Peter N. Horton,d Michael B. ture yields the unusal title compound, bis[isocyano(trimethyl- Hursthouse,d Carl Redshawe and amino)borane]gold(I) diiodoaurate(I), [Au(C4H11BN2)2]- f [AuI ], which forms via an in situ rearrangement of isocyano- Simon M. Humphrey * 2 borane and halide ligands. The structure consists of alternating a + Department of Chemistry and Biochemistry, [L2Au] and [AuI2] ions, which form an in®nite linear one- University of California, Santa Barbara, CA dimensional chain due to aurophilic Au Au interactions. 93106-9510, USA, bUniversitaÈtTuÈbingen, Both Au atoms occupy inversion centres. ÁÁÁ Institut fuÈr Anorganische Chemie, Auf der Morgenstelle 18, D-72076 TuÈbingen, Germany, cChemistry Department, Loughborough University, Loughborough, Leicestershire Comment d LE11 3TU, England, School of Chemistry, We have recently been interested in the formation of University of Southampton, Southampton (isocyanide)gold(I) halide adducts, because of their propen- SO17 1BJ, England, eWolfson Materials and Catalysis Centre, School of Chemical Sciences sity to interact aurophilically. The term aurophilicity is used to and Pharmacy, University of East Anglia, describe observed Au Au interactions.