Fugacity and Activity Analysis of the Bioaccumulation and Environmental Risks of Decamethylcyclopentasiloxane (D5)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
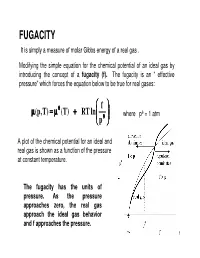
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

The Acidity of Atmospheric Particles and Clouds Havala O
The Acidity of Atmospheric Particles and Clouds Havala O. T. Pye1, Athanasios Nenes2,3, Becky Alexander4, Andrew P. Ault5, Mary C. Barth6, Simon L. Clegg7, Jeffrey L. Collett, Jr.8, Kathleen M. Fahey1, Christopher J. Hennigan9, Hartmut Herrmann10, Maria Kanakidou11, James T. Kelly12, I-Ting Ku8, V. Faye McNeill13, Nicole Riemer14, Thomas 5 Schaefer10, Guoliang Shi15, Andreas Tilgner10, John T. Walker1, Tao Wang16, Rodney Weber17, Jia Xing18, Rahul A. Zaveri19, Andreas Zuend20 1Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC, 27711, USA 2School of Architecture, Civil and Environmental Engineering, Ecole Polytechnique Fédérale de Lausanne, Lausanne, CH- 1015, Switzerland 10 3Institute for Chemical Engineering Sciences, Foundation for Research and Technology Hellas, Patras, GR-26504, Greece 4Department of Atmospheric Science, University of Washington, Seattle, WA, 98195, USA 5Department of Chemistry, University of Michigan, Ann Arbor, MI, 48109-1055, USA 6National Center for Atmospheric Research, Boulder, CO, 80307, USA 7School of Environmental Sciences, University of East Anglia, Norwich NR4 7TJ, UK 15 8Department of Atmospheric Science, Colorado State University, Fort Collins, CO, 80523, USA 9Department of Chemical, Biochemical, and Environmental Engineering, University of Maryland Baltimore County, Baltimore, MD, 21250, USA 10Leibniz Institute for Tropospheric Research (TROPOS), Atmospheric Chemistry Department (ACD), Leipzig, 04318, Germany 20 11Department of Chemistry, University -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Chaotic Temperatures Vs Coefficients of Thermodynamic Activity: the Advantage of the Method of Chemical Dynamics
CHAOTIC TEMPERATURES VS COEFFICIENTS OF THERMODYNAMIC ACTIVITY: THE ADVANTAGE OF THE METHOD OF CHEMICAL DYNAMICS B. Zilbergleyt Bank One, IT Department, Chicago E-mail: [email protected] ABSTRACT. The article compares traditional coefficients of thermodynamic activity as a parameter related to individual chemical species to newly introduced reduced chaotic temperatures as system characteristics, both regarding their usage in thermodynamic simulation of open chemical systems. Logical and mathematical backgrounds of both approaches are discussed. It is shown that usage of reduced chaotic temperatures and the Method of Chemical Dynamics to calculate chemical and phase composition in open chemical systems is much less costly, easier to perform and potentially leads to better precision. PROBABILISTIC MODEL OF COMPLEX CHEMICAL EQUILIBRIUM. It is well known how easily the Guldberg-Waage’s equation can be derived from the probabilistic considerations. Being based on the particle collisions, typical for the reactions in gases, it states that chance of the reaction to happen is proportional to joint probability P(A) of reactant particles to occur simultaneously at the same point of the reaction space. This probability merely equals to product of concentrations of the colliding particles. If multiplied by the rate constant it defines the reaction rate. When the rates of the forward and the reverse reactions are equal then the state of equilibrium exists. Less known is the fact that the above derivation is valid only in the case when no one of reactants can be consumed in any other collision with different outcome than A. Chemical system with only one type of collision represents isolated by the reactants (more traditionally referred to as closed) is a simple chemical system: one type of collision – one outcome. -

Chemical Equilibrium As Balance of the Thermodynamic Forces
Chemical Equilibrium as Balance of the Thermodynamic Forces B. Zilbergleyt, System Dynamics Research Foundation, Chicago, USA, E-mail: [email protected] ABSTRACT. The article sets forth comprehensive basics of thermodynamics of chemical equilibrium as balance of the thermodynamic forces. Based on the linear equations of irreversible thermodynamics, De Donder definition of the thermodynamic force, and Le Chatelier’s principle, our new theory of chemical equilibrium offers an explicit account for multiple chemical interactions within the system. Basic relations between energetic characteristics of chemical transformations and reaction extents are based on the idea of chemical equilibrium as balance between internal and external thermodynamic forces, which is presented in the form of a logistic equation. This equation contains only one new parameter, reflecting the external impact on the chemical system and the system’s resistance to potential changes. Solutions to the basic equation at isothermic-isobaric conditions define the domain of states of the chemical system, including four distinctive areas from true equilibrium to true chaos. The new theory is derived exclusively from the currently recognized ideas of chemical thermodynamics and covers both thermodynamics, equilibrium and non-equilibrium in a unique concept, bringing new opportunities for understanding and practical treatment of complex chemical systems. Among new features one should mention analysis of the system domain of states and the area limits, and a more accurate calculation of the equilibrium compositions. INTRODUCTION. Contemporary chemical thermodynamics is torn apart applying different concepts to traditional isolated systems with true thermodynamic equilibriumi and to open systems with self-organization, loosely described as “far-from-equilibrium” area. -

Molecular Thermodynamics
Chapter 1. The Phase-Equilibrium Problem Homogeneous phase: a region where the intensive properties are everywhere the same. Intensive property: a property that is independent of the size temperature, pressure, and composition, density(?) Gibbs phase rule ( no reaction) Number of independent intensive properties = Number of components – Number of phases + 2 e.g. for a two-component, two-phase system No. of intensive properties = 2 1.2 Application of Thermodynamics to Phase-Equilibrium Problem Chemical potential : Gibbs (1875) At equilibrium the chemical potential of each component must be the same in every phase. i i ? Fugacity, Activity: more convenient auxiliary functions 0 i yi P i xi fi fugacity coefficient activity coefficient fugacity at the standard state For ideal gas mixture, i 1 0 For ideal liquid mixture at low pressures, i 1, fi Psat In the general case Chapter 2. Classical Thermodynamics of Phase Equilibria For simplicity, we exclude surface effect, acceleration, gravitational or electromagnetic field, and chemical and nuclear reactions. 2.1 Homogeneous Closed Systems A closed system is one that does not exchange matter, but it may exchange energy. A combined statement of the first and second laws of thermodynamics dU TBdS – PEdV (2-2) U, S, V are state functions (whose value is independent of the previous history of the system). TB temperature of thermal bath, PE external pressure Equality holds for reversible process with TB = T, PE =P dU = TdS –PdV (2-3) and TdS = Qrev, PdV = Wrev U(S,V) is state function. The group of U, S, V is a fundamental group. Integrating over a reversible path, U is independent of the path of integration, and also independent of whether the system is maintained in a state of internal equilibrium or not during the actual process. -

Equation of State. Fugacities of Gaseous Solutions
ON THE THERMODYNAMICS OF SOLUTIONS. V k~ EQUATIONOF STATE. FUGACITIESOF GASEOUSSOLUTIONS' OTTO REDLICH AND J. N. S. KWONG Shell Development Company, Emeryville, California Received October 15, 1948 The calculation of fugacities from P-V-T data necessarily involves a differentia- tion with respect to the mole fraction. The fugacity rule of Lewis, which in effect would eliminate this differentiation, does not furnish a sufficient approximation. Algebraic representation of P-V-T data is desirable in view of the difficulty of nu- merical or graphical differentiation. An equation of state containing two individ- ual coefficients is proposed which furnishes satisfactory results above the critical temperature for any pressure. The dependence of the coefficients on the composi- tion of the gas is discussed. Relations and methods for the calculation of fugacities are derived which make full use of whatever data may be available. Abbreviated methods for moderate pressure are discussed. The old problem of the equation of state has a practical aspect which has become increasingly important in recent times. The systematic description of gas reactions under high pressure requires information about the fugacities, derived sometimes from extensive data but frequently from nothing more than t,he critical pressure and temperature. For practical purposes a representation of the relation between pressure, vol- ume, and temperature based on two or three individual coefficients is desirable, although a representation of this type satisfies the theorem of corresponding states and therefore cannot be accurate. Since the calculation of fugacities involves a differentiation with respect to the mole fraction, an algebraic repre- sentation by means of an equation of state appears to be desirable. -

Thermodynamic Studies of the Fe-Pt System and “Feo”-Containing Slags for Application Towards Ladle Refining
Thermodynamic Studies of the Fe-Pt System and “FeO”-Containing Slags for Application Towards Ladle Refining Patrik Fredriksson Doctoral Dissertation Stockholm 2003 Royal Institute of Technology Department of Material Science and Engineering Division of Metallurgy Akademisk avhandling som med tillstånd av Kungliga Tekniska Högskolan i Stockholm, framlägges för offentlig granskning för avläggande av Teknologie doktorsexamen, fredagen den 7 November 2003, kl. 10.00 i Kollegiesalen, Administrationsbyggnaden, Kungliga Tekniska Högskolan, Valhallavägen 79 Stockholm ISRN KTH/MSE--03/36--SE+THMETU/AVH ISBN 91-7283-592-3 To Anna ii Abstract In the present work, the thermodynamic activites of iron oxide, denoted as “FeO” in the slag systems Al2O3-“FeO”, CaO-“FeO”, “FeO”-SiO2, Al2O3-“FeO”-SiO2, CaO- “FeO”-SiO2 and “FeO”-MgO-SiO2 were investigated by employing the gas equilibration technique at steelmaking temperatures. The strategy was to expose the molten slag mixtures kept in platinum crucibles for an oxygen potential, determined by a CO/CO2-ratio. A part of the iron reduced from the “FeO” in the slag phase was dissolved into the Pt crucible. In order to obtain the activites of “FeO”, chemical analysis of the quenched slag samples together with thermodynamic information of the binary metallic system Fe-Pt is required. Careful experimental work was carried out by employing a solid-state galvanic cell technique as well as calorimetric measurements in the temperature ranges of 1073-1273 K and 300-1988 K respectively. The outcome of these experiments was incorporated along with previous studies into a CALPHAD-type of thermodynamic assessment performed with the Thermo-Calc™ software. The proposed equilibrium diagram enabled extrapolation to higher temperatures. -

Measurement of Carbon Thermodynamic Activity In
J - carbon flow through sensor membrane 83 MEASUREMENT OF CARBON THERMODYNAMIC 2 ACTIVITY IN SODIUM g , cm" , min" ; t - time, s; GJT - sodium flow rate irr/hour, L/hour; F.A, KOZLOV, Yu.I. ZAGORULKO, Yu.P. KOVALEV, H_ - sensor signal, vol. % CH ; V.V. ALEKSEEV g 4 Institute of Physics and Power Engineering, Gr - decarburizing gas flow rate through sensor ; Obninsk, o Union of Soviet Socialist Republics T,t - t emperature. C; t - sensor t emperature; Gg - sodium flow rate through sensor. INTRODUCTION ABSTRACT Continuous detection of carbon thermodynaraic activity in The report presents the brief outline on system of carbon sodium coolant of energy installations with fast neutron reac- activity detecting system in sodium(SCD), operating on the tors presupposes conducting the following main functions; : carbon - permeable membrane, of the methods and the results of - Control of carburization sodium potential with reference to testing it under the experimental circulating loop conditions. construction materials; The results of carbon activity sensor calibration with the u,s,e - Control of sodium coolant accidental contamination by carbon- of equilibrium samples of XI8H9, Fe -8Ni, Pe ~12Mn materials bearing impurities, for example, as a result of oil leakage from are listed. The behawiour of carbon activity sensor signals in a centrifuginal pump cooling system. sogium under various transitional conditions and hydrodynamic Performing these functions under the monisothermal loop condi- perturbation in the circulating loop, containing carbon bearing tions has specific features which on the one hand depend on impurities in the sodium flow and their deposits on the surfa- construction and operating parameters of detectors and on the ces flushed by sodium, are described. -

Fugacity of Condensed Media
Fugacity of condensed media Leonid Makarov and Peter Komarov St. Petersburg State University of Telecommunications, Bolshevikov 22, 193382, St. Petersburg, Russia ABSTRACT Appearance of physical properties of objects is a basis for their detection in a media. Fugacity is a physical property of objects. Definition of estimations fugacity for different objects can be executed on model which principle of construction is considered in this paper. The model developed by us well-formed with classical definition fugacity and open up possibilities for calculation of physical parameter «fugacity» for elementary and compound substances. Keywords: fugacity, phase changes, thermodynamic model, stability, entropy. 1. INTRODUCTION The reality of world around is constructed of a plenty of the objects possessing physical properties. Du- ration of objects existence – their life time, is defined by individual physical and chemical parameters, and also states of a medium. In the most general case any object, under certain states, can be in one of three ag- gregative states - solid, liquid or gaseous. Conversion of object substance from one state in another refers to as phase change. Evaporation and sublimation of substance are examples of phase changes. Studying of aggregative states of substance can be carried out from a position of physics or on the basis of mathematical models. The physics of the condensed mediums has many practical problems which deci- sion helps to study complex processes of formation and destruction of medium objects. Natural process of object destruction can be characterized in parameter volatility – fugacity. For the first time fugacity as the settlement size used for an estimation of real gas properties, by means of the thermodynamic parities de- duced for ideal gases, was offered by Lewis in 1901. -
![Lecture 4. Thermodynamics [Ch. 2]](https://docslib.b-cdn.net/cover/7153/lecture-4-thermodynamics-ch-2-1457153.webp)
Lecture 4. Thermodynamics [Ch. 2]
Lecture 4. Thermodynamics [Ch. 2] • Energy, Entropy, and Availability Balances • Phase Equilibria - Fugacities and activity coefficients -K-values • Nonideal Thermodynamic Property Models - P-v-T equation-of-state models - Activity coefficient models • Selecting an Appropriate Model Thermodynamic Properties • Importance of thermodyyppnamic properties and equations in separation operations – Energy requirements (heat and work) – Phase equilibria : Separation limit – Equipment sizing • Property estimation – Specific volume, enthalpy, entropy, availability, fugacity, activity, etc. – Used for design calculations . Separator size and layout . AiliAuxiliary components : PiPipi ing, pumps, va lves, etc. EnergyEnergy,, Entropy and Availability Balances Heat transfer in and out Q ,T Q ,T in s out s OfdOne or more feed streams flowing into the system are … … separated into two or more prodttduct streams thtflthat flow Streams in out of the system. n,zi ,T,P,h,s,b,v : Separation : : : process n Molar flow rate : (system) : z Mole fraction Sirr , LW Streams out T Temperature n,zi ,T,P,h,s,b,v P Pressure h Molar enthalpy … … (Surroundings) s Molar entropy T b Molar availability 0 (W ) (W ) s in s out v Specific volume Shaft work in and out Energy Balance • Continuous and steady-state flow system • Kinetic, potential, and surface energy changes are neglected • First law of thermodynamics (conservation of energy) (stream enthalpy flows + heat transfer + shaft work)leaving system - (t(stream en thlthalpy flows +h+ hea ttt trans fer + s hfthaft wor k)entering -

THE CONCEPT of ELECTRON ACTIVITY and ITS RELATION to REDOX POTENTIALS in AQUEOUS GEOCHEMICAL SYSTEMS by Donald C
THE CONCEPT OF ELECTRON ACTIVITY AND ITS RELATION TO REDOX POTENTIALS IN AQUEOUS GEOCHEMICAL SYSTEMS by Donald C. Thorstenson U.S. GEOLOGICAL SURVEY Open-File Report 84 072 1984 UNITED STATES DEPARTMENT OF THE INTERIOR WILLIAM T. CLARK, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report may be write to: purchased from: Regional Research Hydrologist U.S. Geological Survey U.S. Geological Survey Western Distribution Branch Water Resources Division Open-File Services Section 432 National Center Box 25425, Federal Center Reston, Virginia 22092 Denver, Colorado 80225 ERRATA: (p. 1) 1. Equation (2), p. 3, should read: n 2.30RT 1 n 2.30RT , x = Ej? + _____ log__= E° + _____pH . (2) 2. Table 1, p. 6, entry 5, should read: In pure water, p e - 6.9; 3. Line 3, paragraph 3, p. 10 should read: As Fe^+ / aa \ is converted to Fe ( aa ) via equation (15) to 4. Equation (22), p. 13, should read: a n p - anya n+ e (aq) UA (aq) K = . (22) 5. Equation (38), p. 18, should read: F -log ae - = _______ Eh + . (38) ( acl) 2.303RT 2.303RT 6. Equation (52), p. 23, should read: ae- (aq) 7. Equation (53), p. 23, should read: pe s +6.9 and Eh s + 0.41 volt. (53) 8. Equation (54c), p. 23, should read: nu-'e ~ 10 -55.5 (aq) ERRATA: (p. 2) 9. The second line from the bottom, p. 28, should read: ....... (a state that has been maintained for * 105 years based on .... 10. The heading of Table 2, p.