Acid Reduction Techniques in Must and Wine
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Basics of Oak Barrels and Oak Additives
The Basics of Oak Barrels and Oak Additives Presented By: Daniel Brick 231-642-1112 [email protected] Objective To provide the basic and simple information about the selection of oak barrels and oak additives in order to develop an oak program that enhances your wine style and budget. Oak Species Over 250 different oak species throughout the world Over 60 different oak species in the United States Three species used for barrels (wine & spirits) Quercus Alba / American Oak Quercus Petrae / French – Eastern European Quercus Robur / French – Eastern European Predominately used for spirits Very wide grain High tannins / low aromatics Basic Difference Between American and French / Eastern European Oak American Oak has greater levels of whiskey lactones Very high aromatics Very sweet, coconut flavor Bold and aggressive flavors French and Eastern European Oak has two to three times more tannin Wider range of flavors Softer, more elegant American Oak Primary Growing Regions: Missouri Oak Missouri, Illinois, Indiana, W. Kentucky Very sweet, coconut, cloves Bold and aggressive Widest grain of American Oak Used for bourbon Minnesota Oak Minnesota, Wisconsin, N. Iowa, Michigan Tightest grain of American Oak Closest to the French, respectful of the fruit Appalachian Oak Pennsylvania, Virginia, W. Virginia Spice characteristic, pepper, clove Eastern European Oak Similar to French Oak, just different terroir Forests not managed (mixed Petrae and Robur) Grain width critical Aging time depends on grain French Oak Forest Designation: Allier, Troncais, Never, Vosges, Limousin Oak Forests Grain Width vs. Forest: Medium Grain, 3.5 – 2.5 mm, <12 mo. Semi-Fine Grain, 3.0 – 2.0 mm, 8-14 mo. -

WINE CHEM 101 Part B by Bob Peak
WINE CHEM 101 Part B By Bob Peak In last year’s catalog and newsletter, we began a discussion of the chemistry of wine and winemaking— Wine Chem 101, Part A—with details about conversion of sugars to alcohol. (That article is still available at thebeveragepeople. com). At the end of the article, I credited wine acids for the “zing” in wine flavor that lifts it above ordinary bever-ages. So, in this issue, I will tackle that part of Wine Chem: Acid. The two major organic acid components of grapes and grape juice are tartaric and malic acids, usually starting at about a 50-50 ratio. Together, they create the low pH conditions that help make wine a stable beverage and provide the pleasant tartness we all associate with it. The combined range of these acids in fresh grape juice will usually fall between 3 and 15 grams per liter (or 0.3 to 1.5%). Although this wide range of acid levels—measured as TA or Titratable Acidity—can be seen around the world, most North Coast grape juice comes in between 0.4 and 0.7% TA, with about 0.65% preferred. There is also a trace of citric acid in grapes, but it is not a significant contributor to TA. Together, these acids are the “fixed” acids of grape juice, joined in some wines by lactic acid from malolactic fermentation. The term “fixed” is used to distinguish from the spoilage acids of wine, the volatile acids. Those acids—mostly acetic acid—are the products of vinegar fermenta-tion and will introduce unpleasant aromas to wine at very low levels. -

Malolactic Fermentation in Red Wine
Fact Sheet WINEMAKING Malolactic fermentation in red wine Introduction Malolactic fermentation (MLF) is a secondary bacterial fermentation carried out in most red wines. Oenococcus oeni, a member of the lactic acid bacteria (LAB) family, is the main bacterium responsible for conducting MLF, due to its ability to survive the harsh conditions of wine (high alcohol, low pH and low nutrients) and its production of desirable wine sensory attributes. One of the important roles of MLF is to confer microbiological stability towards further metabolism of L-malic acid. Specifically, MLF removes the L-malic acid in wine that can be a carbon source for yeast and bacterial growth, potentially leading to spoilage, spritz and unwanted flavours. MLF can also be conducted in some wines to influence wine style. MLF often occurs naturally after the completion of primary fermentation or can also be induced by inoculation with a selected bacterial strain. Since natural or ‘wild’ MLF can be unpredictable in both time of onset and impact on wine quality, malolactic starter cultures are commonly used. In Australia, almost all red wines undergo MLF and 74% are inoculated with bacterial starter cultures (Nordestgaard 2019). This fact sheet provides practical information for induction of MLF in red wine. A separate fact sheet provides equivalent information for white and sparkling base wine. These should also be read in conjunction with another fact sheet, Achieving successful malolactic fermentation, which gives further practical guidelines for MLF induction, monitoring and management. Updated September 2020 Fact Sheet WINEMAKING Key parameters for a successful MLF in red wine Composition of red wine/must The main wine compositional factors that determine the success of MLF are alcohol, pH, temperature and sulfur dioxide (SO2) concentration. -

No Oak CHARDONNAY 2017 Central Coast
No Oak CHARDONNAY 2017 Central Coast WINEMAKER NOTES The No Oak Chardonnay is a classic representation of a cool climate Chardonnay. This wine is fermented and aged in stainless steel tanks, showcasing the true nature of the varietal. The cool climate shines in this wine’s tension and bright acidity. Malolactic conversion was not encouraged to preserve the freshness, while time on the lees built up the mouthfeel and mid-palate. The long finish is a true testament of its quality and persistence. The beauty of this wine is that it serves many purposes: its backbone of acidity makes for a delicious accompaniment with food and its refreshing personality is perfect to be enjoyed on a warm California day. Anytime is the right tine to drink a wine this attractive and tasty. ABOUT THE VINEYARD The Central Coast of California and its proximity to the Pacific Ocean offers the ideal climate for Chardonnay and is famed as a premier region for quality Chardonnay. The Central Coast vineyards receive a moderating influence from the misty marine layer and refreshing sea breeze. With the cool climate, the Chardonnay grapes experience a long hang time on the vine to achieve optimal ripeness, while maintaining a crisp acidity and fresh aromas. The coastal climate gives rise to a sophisticated and elegant wine with lower alcohol. Color Straw with a touch of a green hue On the Nose Candied apple rings, dried mango, ripe honeydew melon, vanilla blossom and salinity On the Palate Bright acidity start to finish, fresh white peach, Granny Smith apple, wet ABOUT TOLOSA stone minerality and showcasing a rich mid-palate At Tolosa, we are true believers in the special terroir that is Edna Valley, nestled amongst the Blend Chardonnay northwest to southeast running volcanic hills of San Luis Obispo County and the frontier Fermentation Stainless steel tanks for the most complex cool-climate varietals in California. -

Tasting Notes
TASTING NOTES 2019 DRY RIESLING Crisp, refreshing, and bright this wine offers enticing Varietal: 100% Riesling minerality. Pleasantly dry with lively flavors of Mandarin Appellation: Oak Knoll District of Napa Valley peel, green apple, and lemon backed by zesty acidity. The Estate Vineyard: 100% Main Ranch finish is long, mouthwatering and delightfully tart. Harvest: August 30–September 17 94 POINTS (v. 2018) Residual Sugar: 4.6 grams/L (Dry) Wine Enthusiast June 2020 Alcohol: 12.0% 2018 CHARDONNAY Aromas of lemon and lime are complemented by a touch of Varietal: 100% Chardonnay apple blossom and white peach. Subtle notes of toasted oak Appellation: Oak Knoll District of Napa Valley frame the palate which features more citrus flavors and hints Estate Vineyard: 100% Main Ranch of apple and pear. Harvest: August 17–October 1 Oak: 9 months, 13% new 93 POINTS Barrel Fermentation: 61% Wine Enthusiast June 2020 Malolactic Fermentation: 4% Alcohol: 13.3% 2018 MERLOT Aromas of ripe raspberry and plum are layered with earthy Varietal: 90% Merlot, 10% Cabernet Sauvignon spices of black pepper and tea leaves. Concentrated red fruit Appellation: Oak Knoll District of Napa Valley flavors are fresh and bright on the palate leading to expansive Estate Vineyard: 100% Main Ranch notes of spice and forest floor. The finish is well-rounded with Harvest: September 17–October 23 balanced tannins and graceful acidity. Oak: 18 months, 37% new Alcohol: 14.1% 90 POINTS (v. 2017) Wine Enthusiast April 2020 2017 CABERNET SAUVIGNON Ripe, focused flavors of cherry, boysenberry, and Varietal: 87% Cabernet Sauvignon, 4% Merlot, blackcurrant are complemented by soft notes of cedar, bay 4% Cabernet Franc, 3% Malbec, 2% Petit Verdot leaf, and a savory earthiness. -
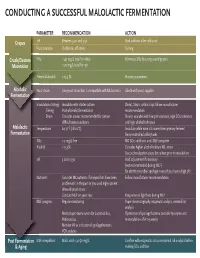
Conducting a Successful Malolactic Fermentation
CONDUCTING A SUCCESSFUL MALOLACTIC FERMENTATION PARAMETERPA RECOMMENDATION ACTION Grapes pH Between 3.20 and 3.50 Acid addition after cold soak FruitFru condition Visible rot, off odors Sorting Crush/Destem SO2SO < 40 mg/L total for white Minimize SO2 by using sound grapes Maceration < 70 mg/L total for red PotentialPPot alcohol < 13.5 % Harvest parameters Alcoholic YeastYeY a strain Use yeast strain that is compatible with ML bacteria Check with yeast supplier Fermentation InoculationIno strategy Inoculate with starter culture Direct, Step 1, or Build up: follow manufacturer Timing Post-alcoholic fermentation recommendation Strain Consider strains recommended for certain Strains available with low pH tolerance, high SO2 tolerance difficult wine conditions and high alcohol tolerance Malolactic TemperatureTem 64-71°F (18-22°C) Inoculate while wine still warm from primary ferment Fermentation Temp-controlled cellar/tanks SO2SO < 5 mg/L free NO SO2 additions until MLF complete AlcoholAlc < 13.5% Consider higher alcohol tolerant ML strain Use acclimatization steps for culture prior to inoculation pH 3.20 to 3.50 Acid adjustment if necessary (not recommended during MLF) Be alert to microbial spoilage issues if you have a high pH NNutrientsu Consider ML nutrients if vineyard lots have been Follow manufacturer recommendation problematic in the past or you used high nutrient demand yeast strain Conduct MLF on yeast lees Keep wine on light lees during MLF MMLFL progress Regular monitoring Paper chromatography, enzymatic analysis, external lab analysis Microscopic examination for Lactobacillus, If presence of spoilage bacteria consider lysozyme and Pediococcus re-inoculation after 2-3 weeks Monitor VA as indicator of spoilage bacteria PCR analysis Post Fermentation MLFML completion Malic acid < 30-50 mg/L Confirm with enzymatic assay or external lab analysis before & Aging making SO2 addition. -

2018 Napa Valley Estate Oak Free Chardonnay
2018 Napa Valley Estate Oak Free Chardonnay St. Supéry Estate Vineyards and Winery is a 100% Estate Grown, sustainably farmed winery lo- cated in the renowned Rutherford growing region in the heart of Napa Valley. Committed to pro- ducing the highest quality estate wines without compromise, we focus on sustainable winery and farming operations to protect the land and environment for future generations. Our Napa Valley Estate collection includes Sauvignon Blanc, Cabernet Sauvignon, Oak Free Chardonnay, Rosé and Moscato. These wines are balanced and showcase bright, fruit-forward flavors. Winemaker’s Notes In the glass, the wine presents a bright, light-yellow straw color with hints of green. Rich tropical aromas combine with apricot, peach and a hint of kiwi. Apricot continues through the palate with ripe nectarine and mandarin orange flavors that are highlighted by lemon zest on the finish, creating a balanced and crisp Chardonnay. Vintage 2018 The 2018 growing season started with a relatively mild winter and spring. There was decent rain in early and mid-January, almost none in February, and a fair amount in March and April which filled our lakes and kicked off the growing season. July was warm and August saw markedly cooler temperatures absent of heat spikes, followed by ideal weather in September and October. There was a little rain in early October, but warm weather and dry breezes followed, allowing the grapes to hang on the vines longer and further develop their flavors. Harvest continued through November because of the ideal weather enabling the fruit to stay on the vine with no pressure from Mother Nature. -

Château Simone
Château Simone This historic estate, situated in the hills just south of Aix-en-Provence, has been in the hands of four generations of the Rougier family since 1830 and holds a virtual monopoly on the appellation of Palette. The property totals 120 hectares, with 28 under vine, of which 23 qualify for the Palette AOC. Its vineyards sit on limestone soils at elevations between 500 and 750 feet on the slopes of the Montaiguet, whose special microclimate is influenced by the encircling pine forests, the mass of the Mont Sainte-Victoire, and the Arc River. The vineyards were reconstituted after phylloxera and many vines are over a century old. In an industry that moves quicker than ever and presents ever-increasing novelty, an estate like Simone is an anchor of meaning and a lens into Provence's patrimony. Viticulture: • Farming: Ecocert Organic Certification Pending. Viticulture has always been practicing organic. • Treatments : No herbicides, chemical fertilizers, or synthetic treatments • Ploughing: Extensive ploughing and working of the soil by tractor. • Soils: Limestone Scree • Vines: Head-trained vines, many over 100-years old, that are replanted on a vine-by-vine basis to maintain a healthy vineyard • Yields: Old vines and extensive debudding lower yields and eliminate the need for a green harvest. • Harvest: Entirely manual harvest, grapes sorted in the vineyard and sorted again in the cellar • Purchasing: Always entirely estate fruit Vinification: Aging: • Fermentation: All wines are fermented spontaneously in large • Élevage: Palette wines are stored in foudres for 18 months; wooden foudres with temperature control for 2 weeks. red and white wines spend an additional year in neutral barrel. -

2018 Oak Knoll District of Napa Valley Merlot
2018 Oak Knoll District of Napa Valley Merlot Vineyard Notes: The Silenus Merlot is a blend of Merlot from the Materra Vineyard and the Silenus Estate Vineyard both located in the heart of the Oak Knoll District of Napa Valley. The OKD is one of the most diverse growing regions in the Napa Valley. The close proximity to the San Pablo Bay provides the fruit with warm days and cool nights. It has a perfect convergence of growing conditions, the mildest climate in the Napa Valley, alluvial fan soils that provide the right balance of nutrients and stress, and the longest growing season, about 8 months, that results in full and complex flavor development in the grapes. The Materra vineyard enjoys especially cool evenings due to its proximity to Napa River and the San Pablo Bay, which is critical for growing rich and complex Merlot. The final blend is completed with Cabernet Sauvignon from our estate vineyard in Oak Knoll District in Napa Valley. Tasting Notes: Bright aromas of black cherry, blackberry jam and oak grace the nose. Dense and lush red fruit flavors of black raspberry and plum are followed by dark chocolate on the mid-palate. The finish is very long and silky with reverberating flavors of black cherry and toasted vanilla. This Merlot is wonderfully balanced with fruit, acidity, and oak all contributing to a classy and delicious wine. Blend: 92.05% Merlot: Materra Vineyard, Oak Knoll 7.95% Cabernet Sauvignon: Estate Vineyards, Oak Knoll All of the wine was aged in 40% new French oak, 10% new American oak (both medium plus and heavy toast), and 50% neutral barrels for 26 months. -

The Clinical Significance of the Organic Acids Test
The Clinical Significance of the Organic Acids Test The Organic Acids Test (OAT) provides an accurate metabolic snapshot of what is going on in the body. Besides offering the most complete and accurate evaluation of intestinal yeast and bacteria, it also provides information on important neurotransmitters, nutritional markers, glutathione status, oxalate metabolism, and much more. The test includes 76 urinary metabolite markers that can be very useful for discovering underlying causes of chronic illness. Patients and physicians report that treating yeast and bacterial abnormalities reduces fatigue, increases alertness and energy, improves sleep, normalizes bowel function, and reduces hyperactivity and abdominal pain. The OAT Assists in Evaluating: ■ Krebs Cycle Abnormalities ■ Neurotransmitter Levels ■ Nutritional Deficiencies ■ Antioxidant Deficiencies ■ Yeast and Clostridia Overgrowth ■ Fatty Acid Metabolism ■ Oxalate Levels ■ And More! The OAT Pairs Well with the Following Tests: ■ GPL-TOX: Toxic Non-Metal Chemical Profile ■ IgG Food Allergy + Candida ■ MycoTOX Profile ■ Phospholipase A2 Activity Test Learn how to better integrate the OAT into your practice, along with our other top tests by attending one of our GPL Academy Practitioner Workshops! Visit www.GPLWorkshops.com for workshop dates and locations. The following pages list the 76 metabolite markers of the Organic Acids Test. Included is the name of the metabolic marker, its clinical significance, and usual initial treatment. INTESTINAL MICROBIAL OVERGROWTH Yeast and Fungal Markers Elevated citramalic acid is produced mainly by Saccharomyces species or Propionibacteria Citramalic Acid overgrowth. High-potency, multi-strain probiotics may help rebalance GI flora. A metabolite produced by Aspergillus and possibly other fungal species in the GI tract. 5-Hydroxy-methyl- Prescription or natural antifungals, along with high-potency, multi-strain probiotics, furoic Acid may reduce overgrowth levels. -

CALCIUM STABILITY in a Nutshell ESSENTIAL CHEMISTRY
CALCIUM STABILITY in a nutshell ESSENTIAL CHEMISTRY Precipitation of calcium tartrate is becoming more frequent all over the world and causing both economic and brand damage that companies should be aware of. The cause of the problem is still unknown but may be found in climate change, viticultural and enological practices or the use of untreated concrete tanks. Whatever the origin, it is important to know how to identify wines that are potentially calcium unstable and how to treat them. Calcium content (0.04 - 0.15 g/L) of wine is 10-20 times lower than potassium. Calcium precipitates mainly as calcium tartrate (CaT). CaT solubility is only 3 times lower at -4°C than at 20°C: cooling has little effect on the rate of CaT precipitation. Potassium bitartrate precipitation does not induce that of CaT. Low calcium content and the presence of inhibiting factors in wine makes the formation of CaT germs that start the crystallization process unpredictable. WINE COMPOUNDS THAT HAVE AN INHIBITING EFFECT ON CALCIUM PRECIPITATION GLUCONIC ACID MALIC ACID CITRIC ACID COLLOIDS POTASSIUM MAGNESIUM MAIN FACTORS PROMOTING CaT PRECIPITATION CALCIUM LOW HIGH INSTABILITY TARTARIC ACID INSTABILITY pH The main factors promoting calcium precipitation are calcium, pH and tartaric acid. In particular, pH has a tremendous impact. The increase of only 0.1 points has a dramatic effect on the speed and intensity of precipitation. HOW TO RECOGNIZE CALCIUM TARTRATE PRECIPITATE Both CaT and potassium bitartrate form white (or red in the case of red wine) crystals and a sandy precipitate. To distinguish one salt from the other, perform the following trial put some crystals in a flask or beaker add some clean water warm the solution between 80-100°C stir occasionally If crystals do not dissolve, it is calcium tartrate ENARTIS SOLUTION FOR CALCIUM STABILITY HOW TO CHECK IF WINE IS CALCIUM UNSTABLE Analyze wine calcium content (Ca1). -

Acetaldehyde Stimulation of Net Gluconeogenic Carbon Movement from Applied Malic Acid in Tomato Fruit Pericarp Tissue'12
Plant Physiol. (1991) 95, 954-960 Received for publication July 18, 1990 0032-0889/91 /95/0954/07/$01 .00/0 Accepted November 16, 1990 Acetaldehyde Stimulation of Net Gluconeogenic Carbon Movement from Applied Malic Acid in Tomato Fruit Pericarp Tissue'12 Anna Halinska3 and Chaim Frenkel* Department of Horticulture, Rutgers-The State University, New Brunswick, New Jersey 08903 ABSTRACT appears to stimulate a respiratory upsurge in climacteric and Applied acetaldehyde is known to lead to sugar accumulation nonclimacteric fruit including blueberry and strawberry (13) in fruit including tomatoes (Lycopersicon esculentum) (O Paz, HW as well as in potato tubers (24) and an enhanced metabolite Janes, BA Prevost, C Frenkel [1982] J Food Sci 47: 270-274) turnover in ripening fig (9). The action of AA may be inde- presumably due to stimulation of gluconeogenesis. This conjec- pendent of ethylene, because AA was shown on one hand to ture was examined using tomato fruit pencarp discs as a test inhibit ethylene biosynthesis (E Pesis, personal communica- system and applied -[U-14C]malic acid as the source for gluco- tion) and on the other to promote softening and degreening neogenic carbon mobilization. The label from malate was re- in pear even when ethylene biosynthesis and action were covered in respiratory C02, in other organic acids, in ethanol arrested ( 14). insoluble material, and an appreciable amount in the ethanol The finding that AA application is accompanied by an soluble sugar fraction. In Rutgers tomatoes, the label recovery in increase in the total sugars content in tomato (19, 21) raises the sugar fraction and an attendant label reduction in the organic acids fraction intensified with fruit ripening.