The in Vitro Neurotoxic and Myotoxic Effects of the Venom from the Suta
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cravens Peak Scientific Study Report
Geography Monograph Series No. 13 Cravens Peak Scientific Study Report The Royal Geographical Society of Queensland Inc. Brisbane, 2009 The Royal Geographical Society of Queensland Inc. is a non-profit organization that promotes the study of Geography within educational, scientific, professional, commercial and broader general communities. Since its establishment in 1885, the Society has taken the lead in geo- graphical education, exploration and research in Queensland. Published by: The Royal Geographical Society of Queensland Inc. 237 Milton Road, Milton QLD 4064, Australia Phone: (07) 3368 2066; Fax: (07) 33671011 Email: [email protected] Website: www.rgsq.org.au ISBN 978 0 949286 16 8 ISSN 1037 7158 © 2009 Desktop Publishing: Kevin Long, Page People Pty Ltd (www.pagepeople.com.au) Printing: Snap Printing Milton (www.milton.snapprinting.com.au) Cover: Pemberton Design (www.pembertondesign.com.au) Cover photo: Cravens Peak. Photographer: Nick Rains 2007 State map and Topographic Map provided by: Richard MacNeill, Spatial Information Coordinator, Bush Heritage Australia (www.bushheritage.org.au) Other Titles in the Geography Monograph Series: No 1. Technology Education and Geography in Australia Higher Education No 2. Geography in Society: a Case for Geography in Australian Society No 3. Cape York Peninsula Scientific Study Report No 4. Musselbrook Reserve Scientific Study Report No 5. A Continent for a Nation; and, Dividing Societies No 6. Herald Cays Scientific Study Report No 7. Braving the Bull of Heaven; and, Societal Benefits from Seasonal Climate Forecasting No 8. Antarctica: a Conducted Tour from Ancient to Modern; and, Undara: the Longest Known Young Lava Flow No 9. White Mountains Scientific Study Report No 10. -

Guidelines for Keeping Venomous Snakes in the NT
GUIDELINES FOR KEEPING VENOMOUS SNAKES IN THE NT Venomous snakes are potentially dangerous to humans, and for this reason extreme caution must be exercised when keeping or handling them in captivity. Prospective venomous snake owners should be well informed about the needs and requirements for keeping these animals in captivity. Permits The keeping of protected wildlife in the Northern Territory is regulated by a permit system under the Territory Parks and Wildlife Conservation Act 2006 (TPWC Act). Conditions are included on permits, and the Parks and Wildlife Commission of the Northern Territory (“PWCNT”) may issue infringement notices or cancel permits if conditions are breached. A Permit to Keep Protected Wildlife enables people to legally possess native vertebrate animals in captivity in the Northern Territory. The permit system assists the PWCNT to monitor wildlife kept in captivity and to detect any illegal activities associated with the keeping of, and trade in, native wildlife. Venomous snakes are protected throughout the Northern Territory and may not be removed from the wild without the appropriate licences and permits. People are required to hold a Keep Permit (Category 1–3) to legally keep venomous snakes in the Northern Territory. Premises will be inspected by PWCNT staff to evaluate their suitability prior to any Keep Permit (Category 1– 3) being granted. Approvals may also be required from local councils, the Northern Territory Planning Authority, and the Department of Health and Community Services. Consignment of venomous snakes between the Northern Territory and other States and Territories can only be undertaken with an appropriate import / export permit. There are three categories of venomous snake permitted to be kept in captivity in the Northern Territory: Keep Permit (Category 1) – Mildly Dangerous Venomous Keep Permit (Category 2) – Dangerous Venomous Keep Permit (Category 3) – Highly Dangerous Venomous Venomous snakes must be obtained from a legal source (i.e. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Fowlers Gap Biodiversity Checklist Reptiles
Fowlers Gap Biodiversity Checklist ow if there are so many lizards then they should make tasty N meals for someone. Many of the lizard-eaters come from their Reptiles own kind, especially the snake-like legless lizards and the snakes themselves. The former are completely harmless to people but the latter should be left alone and assumed to be venomous. Even so it odern reptiles are at the most diverse in the tropics and the is quite safe to watch a snake from a distance but some like the Md rylands of the world. The Australian arid zone has some of the Mulga Snake can be curious and this could get a little most diverse reptile communities found anywhere. In and around a disconcerting! single tussock of spinifex in the western deserts you could find 18 species of lizards. Fowlers Gap does not have any spinifex but even he most common lizards that you will encounter are the large so you do not have to go far to see reptiles in the warmer weather. Tand ubiquitous Shingleback and Central Bearded Dragon. The diversity here is as astonishing as anywhere. Imagine finding six They both have a tendency to use roads for passage, warming up or species of geckos ranging from 50-85 mm long, all within the same for display. So please slow your vehicle down and then take evasive genus. Or think about a similar diversity of striped skinks from 45-75 action to spare them from becoming a road casualty. The mm long! How do all these lizards make a living in such a dry and Shingleback is often seen alone but actually is monogamous and seemingly unproductive landscape? pairs for life. -

Berriquin LWMP Wildlife
Berriquin Wildlife Murray Land & Water Management Plan Wildlife Survey 2005-2006 Matthew Herring David Webb Michael Pisasale INTRODUCTION Why do a wildlife survey? 106 farms and were surveyed One of the great things about between June 2005 and March living in rural Australia is all the 2006. They incorporated a range wildlife that we share the land- of vegetation types (e.g. Black scape with. Historically, humans Box Woodland) as well as reveg- have impacted on the survival of etation on previously cleared many native plants and animals. land and constructed wetlands. Fortunately, there is a grow- Methods used to survey wildlife ing commitment in the country included: to wildlife conservation on the farm. As we improve our knowl- - Bird surveys edge and understanding of the - Log rolling for reptiles and local landscape and the animals frogs and plants that live in it we will - Spotlighting for mammals, rep be in a much better position to tiles and nocturnal birds conserve and enhance our natu- - Elliot traps for small mammals ral heritage for future genera- and reptiles tions. - Pitfall trapping for reptiles and frogs This wildlife survey was an ini- - Harp traps for bats tiative of the Berriquin Land & - Using the “Anabat” to record Water Management Plan (LWMP) bat calls M.Herring Working Group and is the largest - Call broadcasting to attract Wildlife expert Adam Bester and most extensive ever un- birds with 11 Little Forest Bats, one dertaken in the area. Berriquin of Berriquin’s most abundant was one of four LWMP areas that Other targeted methods were mammals. -

Action Statement Floraflora and and Fauna Fauna Guarantee Guarantee Act Act 1988 1988 No
Action Statement FloraFlora and and Fauna Fauna Guarantee Guarantee Act Act 1988 1988 No. No. ### 108 Hooded Scaly-foot Pygopus nigriceps Description and Distribution The Hooded Scaly-foot Pygopus nigriceps belongs to the reptile family Pygopodidae, the legless or flap-footed lizards. Legless lizards are superficially snake-like; they lack forelimbs, and the hind limbs are reduced to a scaly flap just above the vent. Whilst their eyes are lidless and snake-like, there are several features that distinguish legless lizards from snakes. Most legless lizards have an obvious ear aperture, lacking in all snakes, and a broad fleshy tongue, compared to the deeply forked tongue of snakes. Most legless lizards also have a tail that, when unbroken, is considerably longer than their body. In contrast, the tail of snakes is considerably Hooded Scaly-foot, Pygopus nigriceps shorter than their body. The genus Pygopus Illustration by Peter Robertson Wildlife Profiles P/L © differs from other legless lizards on the basis of the combination of the following features: head covered with enlarged, symmetrical scales; smooth (compared to keeled) ventral scales; and the possession of eight or more preanal pores. Two species of Pygopus occur in Victoria. The Hooded Scaly-foot is a large legless lizard, attaining a total length of 475mm, and a snout- vent length of about 180mm. Females reach larger sizes than males. Variable in colour, the Hooded Scaly-foot may be pale grey to reddish-brown on the dorsal surface and whitish on the ventral surface. The dorsal scales may be dark-edged, forming a reticulated pattern, or individual pale and dark scales may form a vague longitudinal pattern. -

An Investigation of the Evolution of Australian Elapid Snake Venoms
toxins Article Rapid Radiations and the Race to Redundancy: An Investigation of the Evolution of Australian Elapid Snake Venoms Timothy N. W. Jackson 1, Ivan Koludarov 1, Syed A. Ali 1,2, James Dobson 1, Christina N. Zdenek 1, Daniel Dashevsky 1, Bianca op den Brouw 1, Paul P. Masci 3, Amanda Nouwens 4, Peter Josh 4, Jonathan Goldenberg 1, Vittoria Cipriani 1, Chris Hay 1, Iwan Hendrikx 1, Nathan Dunstan 5, Luke Allen 5 and Bryan G. Fry 1,* 1 Venom Evolution Lab, School of Biological Sciences, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (T.N.W.J.); [email protected] (I.K.); [email protected] (S.A.A.); [email protected] (J.D.); [email protected] (C.N.Z.); [email protected] (D.D.); [email protected] (B.o.d.B.); [email protected] (J.G.); [email protected] (V.C.); [email protected] (C.H.); [email protected] (I.H.) 2 HEJ Research Institute of Chemistry, International Centre for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi 75270, Pakistan 3 Princess Alexandra Hospital, Translational Research Institute, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] 4 School of Chemistry and Molecular Biosciences, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (A.N.); [email protected] (P.J.) 5 Venom Supplies, Tanunda, South Australia 5352, Australia; [email protected] (N.D.); [email protected] (L.A.) * Correspondence: [email protected]; Tel.: +61-4-0019-3182 Academic Editor: Nicholas R. -

Recent Taxonomic Changes and Additions to the Snake Fauna of New
21 assessments had been undertaken that did Recent taxonomic changes and additions not result in an application for an agreement or to the snake fauna of New South Wales a statement. 24 assessments were currently being Steve Sass1,2 undertaken, of which: 1EnviroKey, PO Box 7231, Tathra NSW 2550 5 would definitely result in an application 2Ecology & Biodiversity Group, Charles Sturt University, 9 would definitely not result in an application Thurgoona, NSW 2541 10 were undecided/not sure [email protected] To a significant degree, the future of the BioBanking program is in our hands. As Assessors, it is our role to Since the ‘Complete Guide to the Reptiles of introduce the idea to our clients and sell the concept. Australia‛ was first published in 2003, more than 80 No matter how cynical you might be about the reptile species have been added to the list of described modelling, the data upon which it is built, access to reptile species in Australia, bringing the total number the program, the cost of training or the unusual to 923 in the third and most recent addition (Wilson application of the program in part of western Sydney: and Swan 2010). These additions being the result of you must admit that it provides a mechanism to get newly discovered species, naming of previously important privately-owned pieces of country into a undescribed species, and taxonomic reviews of perpetual reserve network. If it is not achieving that, various species and genera. This has resulted in then it is partly our fault and we need to work at it. -
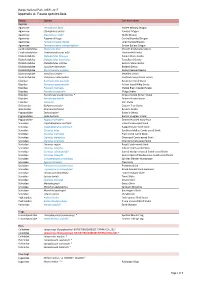
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

Reptiles and Frogs of Gluepot Reserve (Updated June 2020)
Reptiles and Frogs of Gluepot Reserve (updated June 2020) Scientific name Common name Notes with reference to Gluepot Family Agamidae Dragon Lizards Ctenophorus spinodomus1 Eastern Mallee Dragon Terrestrial, associated with spinifex, commonly observed Ctenophorus pictus Painted Dragon Terrestrial, commonly observed Diporiphora nobbi Nobbi Dragon Terrestrial/arboreal, commonly observed Pogona vitticeps Central Bearded Dragon Terrestrial, commonly observed Family Gekkonidae Typical Geckos Gehyra versicolor2 Eastern Tree Dtella Arboreal, nocturnal, commonly observed on buildings Heteronotia binoei Bynoe’s Gecko Terrestrial, nocturnal, occasionally observed Family Carphodactylidae Knob-tails & others Nephrurus levis Smooth Knob-tailed Gecko Terrestrial, nocturnal, occasionally observed Family Diplodactylidae Diplodactylus furcosis Ranges Stone Gecko Terrestrial, nocturnal, occasionally observed Diplodactylus vittatus Eastern Stone Gecko Terrestrial, nocturnal, occasionally observed Lucasium damaeum Beaded Gecko Terrestrial, nocturnal, commonly observed Oedura cincta3 Inland Marbled Velvet Gecko Arboreal, nocturnal, on Black Oak, rarely observed Rhynchoedura angusta4 Border Beaked Gecko Terrestrial, nocturnal, occasionally observed. Strophurus elderi Jewelled Gecko Terrestrial, nocturnal, in spinifex, occasionally observed Strophurus williamsi Eastern Spiny-tailed Gecko Terrestrial/arboreal, nocturnal, occasionally observed Family Pygopodidae Legless Lizards Aprasia inaurita Red-tailed Worm Lizard Terrestrial, rarely observed, often under -

Some New Small-Eyed Snakes from Australia and New Guinea (Serpentes:Elapidae)
Australasian Journal of Herpetology 3 Australasian Journal of Herpetology 13:3-7. ISSN 1836-5698 (Print) ISSN 1836-5779 (Online) Published 30 June 2012. Some new small-eyed snakes from Australia and New Guinea (Serpentes:Elapidae). Raymond T. Hoser 488 Park Road, Park Orchards, Victoria, 3134, Australia. Phone: +61 3 9812 3322 Fax: 9812 3355 E-mail: [email protected] Received 12 March 2012, Accepted 8 April 2012, Published 30 June 2012. ABSTRACT The so-called Small-eyed Snakes from Australia and New Guinea, within the elapid tribe Sutini have had a checkered taxonomic history. Most described species have been shuf- fled between genera by authors sometimes with little apparent concern for rules of nomen- clature and priority. This paper sets out the appropriate genera for the group and species within, based on the relevant rules of the ICZN. Two well-known species that have not been formally described to date are named and diagnosed according to the Zoological Code. Likewise for a subspecies of another taxon. This paper also formally names the previously unnamed eastern subspecies of the Bardick Echiopsis curta. Keywords: Taxonomic revision; new species; Sutini; Cryptophis; Parasuta; Suta; Hulimkai; Rhinoplocephalus; Unechis; Echiopsis; nigrescens; assimilis; boschmai; nigrostriata; edwardsi; crutchfieldi; durhami; curta; martinekae. INTRODUCTION The so-called Small-eyed snakes within Australia have been identification manuals of the time period, including Cogger (1975 placed in various genera by various authors. They are known et. seq. to 2000), Cogger et. al. (1983), Hoser (1989), O’Shea from most parts of mainland Australia and Southern New (1996), Storr, Smith and Johnstone (1986, 2002), Wilson and Guinea. -

Appendix J Biological and Fauna Surveys of the Proposed Development Area
APPENDIX J BIOLOGICAL AND FAUNA SURVEYS OF THE PROPOSED DEVELOPMENT AREA Appendix J - Biological and Fauna Surveys of the Proposed Development Area ORD SUGAR PROJECT Biological Surveys of the Proposed Development Area - May and June 1999 Prepared for: WESFARMERS LIMITED, MARUBENI CORPORATION AND THE WATER CORPORATION OF WESTERN AUSTRALIA Level 7, 40 The Esplanade Perth WA 6000 Prepared by: Kinhill Pty Ltd ACN 007 660 317 47 Burswood Road, Victoria Park WA 6100 Telephone 08 9362 5900, Facsimile 08 9362 5627 August 1999 PV8009-FC-008 Rev. 0 Wesfarmers Ltd, Marubeni Corporation and Water Corporation of Western Australia 1999 Limitations Statement In preparing this report, Kinhill Pty Ltd has relied upon and presumed accurate certain information (or absence thereof) relative to the site that was obtained from examination of records in the public domain or provided by government officials and authorities. Except as otherwise stated in the report, Kinhill has not attempted to verify the accuracy or completeness of any such information. The findings, observations and conclusions expressed by Kinhill Pty Ltd in this report are not, and should not be considered, an opinion concerning the feasibility of establishing irrigated agriculture on the site. No warranty or guarantee, whether express or implied, is made with respect to the data reported or to the findings, observations and conclusions expressed in this report. Further, such data, findings, observations and conclusions are based solely upon information in existence at the time of the investigation. This report has been prepared on behalf of and for the exclusive use of the Wesfarmers Limited, Marubeni Corporation and the Water Corporation of Western Australia, and is subject to and issued in connection with the provisions of the agreement with Kinhill Pty Ltd.