Density Corrections for Moist Air and Other Gases Introduction a Surface Film of Water Can Assume When Evaporating Freely Into the Air
Total Page:16
File Type:pdf, Size:1020Kb
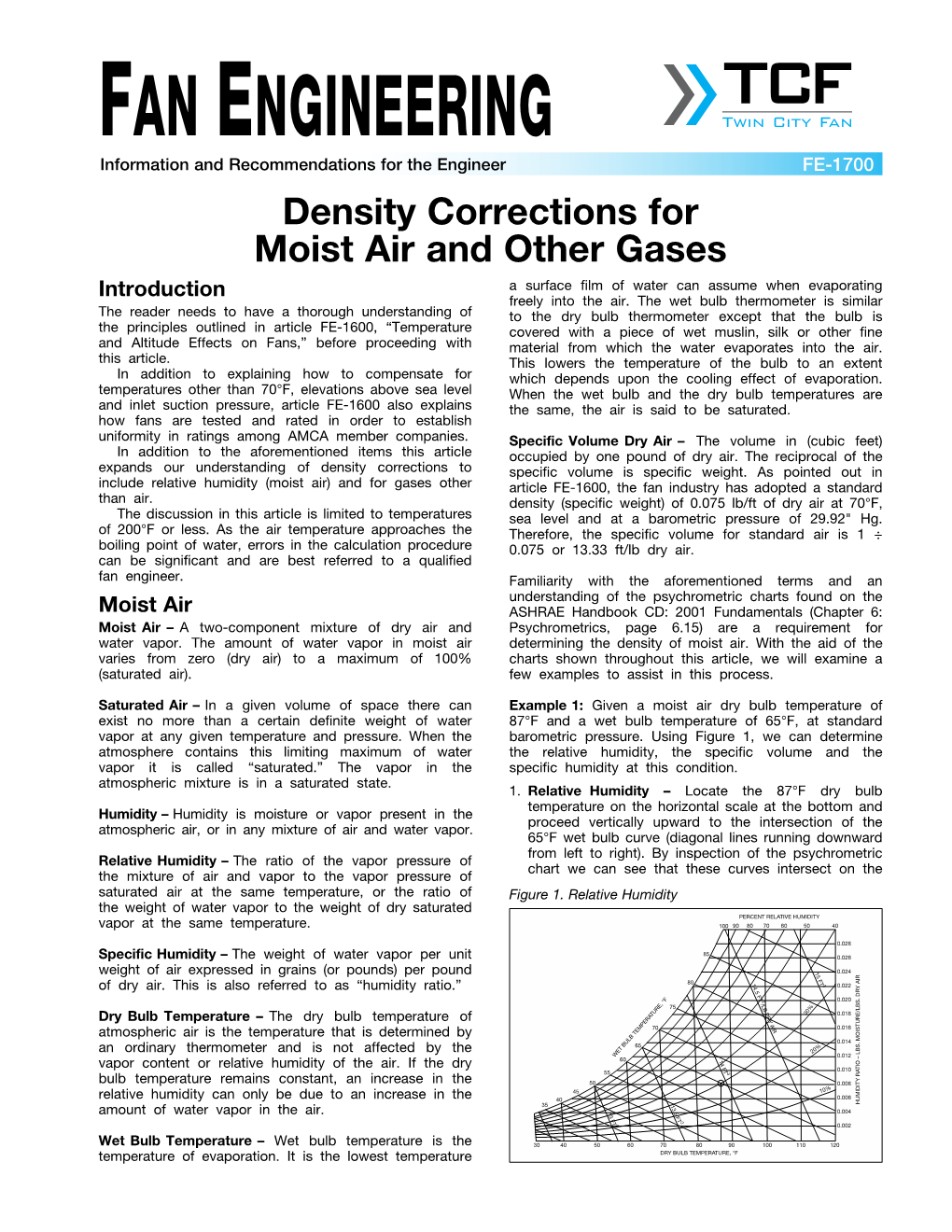
Load more
Recommended publications
-

Density and Specific Weight Estimation for the Liquids and Solid Materials
Laboratory experiments Experiment no 2 – Density and specific weight estimation for the liquids and solid materials. 1. Theory Density is a physical property shared by all forms of matter (solids, liquids, and gases). In this lab investigation, we are mainly concerned with determining the density of solid objects; both regular-shaped and irregular-shaped. In general regular-shaped solid objects are those that have straight sides that can be measured using a metric ruler. These shapes include but are not limited to cubes and rectangular prisms. In general, irregular-shaped solid objects are those that do not have straight sides that cannot be measured with a metric ruler or slide caliper. The density of a material is defined as its mass per unit volume. The symbol of density is ρ (the Greek letter rho). m kg (1) V m3 The specific weight (also known as the unit weight) is the weight per unit volume of a material. The symbol of specific weight is γ (the Greek letter Gamma). W N (2) V m3 On the surface of the Earth, the weight W of an object is related to its mass m by: W = m · g, (3) where g is the acceleration due to the Earth's gravity, equal to about 9.81 ms-2. Using eq. 1, 2 and 3 we will obtain dependence between specific weight of the body and its density: g (4) Apparatus: Vernier caliper, balance with specific gravity platform (additional table in our case), 250 ml graduate beaker. Unknowns: a) Various solid samples (regular and irregular shaped) b) Light liquid sample (alcohol-water mixture) or heavy liquid sample (salt-water mixture). -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B. -

Fluid Mechanics
I. FLUID MECHANICS I.1 Basic Concepts & Definitions: Fluid Mechanics - Study of fluids at rest, in motion, and the effects of fluids on boundaries. Note: This definition outlines the key topics in the study of fluids: (1) fluid statics (fluids at rest), (2) momentum and energy analyses (fluids in motion), and (3) viscous effects and all sections considering pressure forces (effects of fluids on boundaries). Fluid - A substance which moves and deforms continuously as a result of an applied shear stress. The definition also clearly shows that viscous effects are not considered in the study of fluid statics. Two important properties in the study of fluid mechanics are: Pressure and Velocity These are defined as follows: Pressure - The normal stress on any plane through a fluid element at rest. Key Point: The direction of pressure forces will always be perpendicular to the surface of interest. Velocity - The rate of change of position at a point in a flow field. It is used not only to specify flow field characteristics but also to specify flow rate, momentum, and viscous effects for a fluid in motion. I-1 I.4 Dimensions and Units This text will use both the International System of Units (S.I.) and British Gravitational System (B.G.). A key feature of both is that neither system uses gc. Rather, in both systems the combination of units for mass * acceleration yields the unit of force, i.e. Newton’s second law yields 2 2 S.I. - 1 Newton (N) = 1 kg m/s B.G. - 1 lbf = 1 slug ft/s This will be particularly useful in the following: Concept Expression Units momentum m! V kg/s * m/s = kg m/s2 = N slug/s * ft/s = slug ft/s2 = lbf manometry ρ g h kg/m3*m/s2*m = (kg m/s2)/ m2 =N/m2 slug/ft3*ft/s2*ft = (slug ft/s2)/ft2 = lbf/ft2 dynamic viscosity µ N s /m2 = (kg m/s2) s /m2 = kg/m s lbf s /ft2 = (slug ft/s2) s /ft2 = slug/ft s Key Point: In the B.G. -

Glossary of Terms — Page 1 Air Gap: See Backflow Prevention Device
Glossary of Irrigation Terms Version 7/1/17 Edited by Eugene W. Rochester, CID Certification Consultant This document is in continuing development. You are encouraged to submit definitions along with their source to [email protected]. The terms in this glossary are presented in an effort to provide a foundation for common understanding in communications covering irrigation. The following provides additional information: • Items located within brackets, [ ], indicate the IA-preferred abbreviation or acronym for the term specified. • Items located within braces, { }, indicate quantitative IA-preferred units for the term specified. • General definitions of terms not used in mathematical equations are not flagged in any way. • Three dots (…) at the end of a definition indicate that the definition has been truncated. • Terms with strike-through are non-preferred usage. • References are provided for the convenience of the reader and do not infer original reference. Additional soil science terms may be found at www.soils.org/publications/soils-glossary#. A AC {hertz}: Abbreviation for alternating current. AC pipe: Asbestos-cement pipe was commonly used for buried pipelines. It combines strength with light weight and is immune to rust and corrosion. (James, 1988) (No longer made.) acceleration of gravity. See gravity (acceleration due to). acid precipitation: Atmospheric precipitation that is below pH 7 and is often composed of the hydrolyzed by-products from oxidized halogen, nitrogen, and sulfur substances. (Glossary of Soil Science Terms, 2013) acid soil: Soil with a pH value less than 7.0. (Glossary of Soil Science Terms, 2013) adhesion: Forces of attraction between unlike molecules, e.g. water and solid. -

Kozeny-Carman Equation and Hydraulic Conductivity of Compacted Clayey Soils
Geomaterials, 2012, 2, 37-41 http://dx.doi.org/10.4236/gm.2012.22006 Published Online April 2012 (http://www.SciRP.org/journal/gm) Kozeny-Carman Equation and Hydraulic Conductivity of Compacted Clayey Soils Emmanouil Steiakakis, Christos Gamvroudis, Georgios Alevizos Department of Mineral Resources Engineering, Technical University of Crete, Chania, Greece Email: [email protected] Received January 24, 2012; revised February 27, 2012; accepted March 10, 2012 ABSTRACT The saturated hydraulic conductivity of a soil is the main parameter for modeling the water flow through the soil and determination of seepage losses. In addition, hydraulic conductivity of compacted soil layers is critical component for designing liner and cover systems for waste landfills. Hydraulic conductivity can be predicted using empirical relation- ships, capillary models, statistical models and hydraulic radius theories [1]. In the current research work the reliability of Kozeny-Carman equation for the determination of the hydraulic conductivity of compacted clayey soils, is evaluated. The relationship between the liquid limit and the specific surface of the tested samples is also investigated. The result- ing equation gives the ability for quick estimation of specific surface and hydraulic conductivity of the compacted clayey samples. The results presented here show that the Kozeny-Carman equation provides good predictions of the hydraulic conductivity of homogenized clayey soils compacted under given compactive effort, despite the consensus set out in the literature. Keywords: Hydraulic Conductivity; Kozeny-Carman Equation; Specific Surface; Clayey Soils 1. Introduction Chapuis and Aubertin [1] stated that for plastic - cohe- sive soils, the specific surface can be estimated from the As stated by Carrier [2], about a half-century ago Kozeny liquid limit (LL), obtained by standard Casagrande’s and Carman proposed the below expression for predict- method. -

Glossary of Notations
108 GLOSSARY OF NOTATIONS A = Earthquake peak ground acceleration. IρM = Soil influence coefficient for moment. = A0 Cross-sectional area of the stream. K1, K2, = aB Barge bow damage depth. K3, and K4 = Scour coefficients that account for the nose AF = Annual failure rate. shape of the pier, the angle between the direction b = River channel width. of the flow and the direction of the pier, the BR = Vehicular braking force. streambed conditions, and the bed material size. = BRa Aberrancy base rate. Kp = Rankine coefficient. = = bx Bias of ¯x x/xn. KR = Pile flexibility factor, which gives the relative c = Wind analysis constant. stiffness of the pile and soil. C′=Response spectrum modeling parameter. L = Foundation depth. = CE Vehicular centrifugal force. Le = Effective depth of foundation (distance from = CF Cost of failure. ground level to point of fixity). = CH Hydrodynamic coefficient that accounts for the effect LL = Vehicular live load. of surrounding water on vessel collision forces. LOA = Overall length of vessel. = CI Initial cost for building bridge structure. LS = Live load surcharge. = Cp Wind pressure coefficient. max(x) = Maximum of all possible x values. = CR Creep. M = Moment capacity. = cap CT Expected total cost of building bridge structure. M = Moment capacity of column. = col CT Vehicular collision force. M = Design moment. = design CV Vessel collision force. n = Manning roughness coefficient. = D Diameter of pile or column. N = Number of vessels (or flotillas) of type i. = i DC Dead load of structural components and nonstructural PA = Probability of aberrancy. attachments. P = Nominal design force for ship collisions. DD = Downdrag. B P = Base wind pressure. -

What Is Fluid Mechanics?
What is Fluid Mechanics? A fluid is either a liquid or a gas. A fluid is a substance which deforms continuously under the application of a shear stress. A stress is defined as a force per unit area Next, what is mechanics? Mechanics is essentially the application of the laws of force and motion. Fluid statics or hydrostatics is the study of fluids at rest. The main equation required for this is Newton's second law for non-accelerating bodies, i.e. ∑ F = 0 . Fluid dynamics is the study of fluids in motion. The main equation required for this is Newton's second law for accelerating bodies, i.e. ∑ F = ma . Mass Density, ρ, is defined as the mass of substance per unit volume. ρ = mass/volume Specific Weight γ, is defined as the weight per unit volume γ = ρ g = weight/volume Relative Density or Specific Gravity (S.g or Gs) S.g (Gs) = ρsubstance / ρwater at 4oC Dynamic Viscosity, µ µ = τ / (du/dy) = (Force /Area)/ (Velocity/Distance) Kinematic Viscosity, v v = µ/ ρ Primary Dimensions In fluid mechanics there are only four primary dimensions from which all other dimensions can be derived: mass, length, time, and temperature. All other variables in fluid mechanics can be expressed in terms of {M}, {L}, {T} Quantity SI Unit Dimension Area m2 m2 L2 Volume m3 m3 L3 Velocity m/s ms-1 LT-1 Acceleration m/s2 ms-2 LT-2 -Angular velocity s-1 s-1 T-1 -Rotational speed N Force kg m/s2 kg ms-2 M LT-2 Joule J energy (or work) N m, kg m2/s2 kg m2s-2 ML2T-2 Watt W Power N m/s Nms-1 kg m2/s3 kg m2s-3 ML2T-3 Pascal P, pressure (or stress) N/m2, Nm-2 kg/m/s2 kg m-1s-2 ML-1T-2 Density kg/m3 kg m-3 ML-3 N/m3 Specific weight kg/m2/s2 kg m-2s-2 ML-2T-2 a ratio Relative density . -

Fluid Mechanics
FLUID MECHANICS PROF. DR. METİN GÜNER COMPILER ANKARA UNIVERSITY FACULTY OF AGRICULTURE DEPARTMENT OF AGRICULTURAL MACHINERY AND TECHNOLOGIES ENGINEERING 1 3-FLUID STATICS 3.8. Hydrostatic Force on a Curved Surface For a submerged curved surface, the determination of the resultant hydrostatic force is more involved since it typically requires the integration of the pressure forces that change direction along the curved surface. The concept of the pressure prism in this case is not much help either because of the complicated shapes involved. Consider a curved portion of the swimming pool shown in Fig.3.30a. We wish to find the resultant fluid force acting on section BC (which has a unit length perpendicular to the plane of the paper) shown in Fig.3.30b. We first isolate a volume of fluid that is bounded by the surface of interest, in this instance section BC, the horizontal plane surface AB, and the vertical plane surface AC. The free- body diagram for this volume is shown in Fig. 3.30c. The magnitude and location of forces F1 and F2 can be determined from the relationships for planar surfaces. The weight, W, is simply the specific weight of the fluid times the enclosed volume and acts through the center of gravity (CG) of the mass of fluid contained within the volume. Figure 3.30. Hydrostatic force on a curved surface. The forces FH and FV represent the components of the force that the tank exerts on the fluid. In order for this force system to be in equilibrium, the horizontal component must be equal in magnitude and collinear with F2, and the vertical 2 component FV equal in magnitude and collinear with the resultant of the vertical forces F1 and W. -

FAQ-Lecture 2
LECTURE 2- PROPERTIES OF FLUIDS FREQUENTLY ASKED QUESTIONS 1. What are the differences between a liquid and a gas Liquids Gases The molecules of a liquids are much less The molecules of a gas are much further further apart than those of a gas apart than those of a liquid. A liquid is relatively incompressible, and A gas is very compressible, and when all if all the pressure, except that of its own external pressure is removed, it tends to vapourpressure, is removed, the cohesion expand indefinitely between molecules hold them together, so that the liquid does not expand indefinitely The volume of a liquid isnot greatly The volume of a gas or vapouris greatly affected by changes in pressure or affected by changes in pressure or temperature or both. temperature or both. They have definite mass and volume but They have definite mass, but no volume no definite shape. and no definite shape 2. Define the terms specific density, specific weight and specific gravity Density (ρ): It is defined as mass per unit volume. Specific weight ( ): It is defined as weight per unit volume. Specific weight Specific gravity (SG): Specific gravity of a given fluid is defined as the specific weight of the fluid divided by the specific weight of water. Specific gravity of a liquid is the dimensionless ratio 3. Differentiate between absolute and gauge pressure Atmospheric Pressure is the force per unit area exerted against a surface by the weight of air above that surface in the Earth's atmosphere. Gauge Pressure and Absolute Pressure: Gauge pressures are measured relative to the atmosphere, whereas absolute pressures are measured relative to perfect vacuum such as that existing in outer space. -

Air and Its Composition
LECTURE – 1 THE CONTENTS OF THIS LECTURE ARE AS FOLLOWS: 1.0 GENERAL COMPOSITION 1.1 Composition of Dry Air 1.2 Requirement of Sufficient Quantity of Air in Mines 2.0 IMPURITIES IN MINE AIR 3.0 THRESHOLD LIMIT VALUES 3.1 Threshold Limit Values of Gas Mixtures 4.0 RELATIVE DENSITY AND SPECIFIC GRAVITY OF GASES REFERENCES Page 1 of 9 1.0 GENERAL COMPOSITION Let me tell you that air is a non-homogeneous mixture of different gases. There are two ways by which we can represent the composition of air: i) Percentage of gas by volume ii) Percentage of the gas by mass 1.1 Composition of Dry Air The composition of dry air at sea level is given in Table 1. Table 1 General composition of air (Bolz & Tuve, 1973) S.No. Gas Volume Mass Molecular Molecular (%) (%) weight(kg/kmol) weight in air 1. Nitrogen 78.03 75.46 28.015 21.88 2. Oxygen 20.99 23.19 32.00 6.704 3. Carbon 0.03 0.05 44.003 0.013 dioxide 4. Hydrogen 0.01 0.0007 2.016 0 5. Monatomic 0.94 1.30 39.943 0.373 gases(Ar, Rn, He,Kr, Ne) TOTAL 100.00 100.00 28.97 It is important to note that, the composition of different gases (in dry air) by mass is a fixed one whereas the percentage composition of the gases by volume or mass in wet air i.e., air containing moisture is dependent on humidity or the moisture in the air. -
![Chapter1-Fluid and Their Properties [Compatibility Mode]](https://docslib.b-cdn.net/cover/8474/chapter1-fluid-and-their-properties-compatibility-mode-4498474.webp)
Chapter1-Fluid and Their Properties [Compatibility Mode]
CHAPTER (1) Fluids and their Properties Dr. Yunes Mogheir ١ OBJECTIVESOFTHIS CHAPTER: ¢ Define the nature of a fluid. ¢ Show where fluid mechanics concepts are used with those of solid mechanics and indicate some fundamental areas of difference. ¢ Introduce viscosity and show what are Newtonian and non-Newtonian fluids ¢ Define the appropriate physical properties and show how these allow differentiation between solids and fluids as well as between liquids and gases. ٢ 1.1 FLUIDS: ¢ We normally recognize three states of matter: § Solid. § Liquid. § Gas. ¢ However, liquid and gas have common characteristic and both called fluids. ¢ In contrast to solids they lack the ability to resist deformation. ¢ They flow (move) under the action of such forces, and deforms continuously as the force is applied (and the shape of fluids change continuously as long as a force acts). ¢ A solid can resist deformation, this force may cause some displacement but the solid does not continue to move indefinitely. ٣ 1.1 FLUIDS: (CONT.) ¢ The deformation is caused by shearing forces which act tangentially to a surface. ¢ F acting tangentially on a rectangular (solid lined) element ABDC. This is a shearing force and produces the (dashed lined) element A'B'DC. 1.1 FLUIDS: (CONT.) ¢ A Fluid is a substance which deforms continuously, or flows, when subjected to shearing forces. ¢ If a fluid is at rest there are no shearing forces acting. All forces must be perpendicular to the planes which they are acting. Fluid Flow Shear stress –Yes Fluid Rest Shear stress –No ٥ 1.1 FLUIDS: (CONT.) There are two aspects of fluid mechanics which make it different to solid mechanics: § The nature of fluid is much different to that of solid § In fluids we usually deal with continuous streams of fluid without a beginning or end. -

Fluid Mechanics
FLUID MECHANICS PROF. DR. METİN GÜNER COMPILER ANKARA UNIVERSITY FACULTY OF AGRICULTURE DEPARTMENT OF AGRICULTURAL MACHINERY AND TECHNOLOGIES ENGINEERING 1 2. PROPERTIES OF FLUIDS 2.1. Measures of Fluid Mass and Weight Mass: Mass (m) is a scientific measure of the amount of matter an object is made up of. No matter where you are at given moment in time, mass is constant. So, whether you're walking on the world or on the moon, your mass is the same. -Mass is indestructible. No matter where you are in the universe your mass will never change -Mass can never be zero. What we mean by this is that everything in the universe has mass. -Mass is not related to gravity, centrifugal force, etc and these forces have no effect whatsoever on your mass. -Mass is commonly measured in kilograms and grams. Weight: Weight (W) is a form of measurement that is dependent on gravity and, unlike mass, your weight can vary depending on where you are in the universe. W=mg -The weight of an object changes based on where it is. If you've decided to visit the moon to test out this theory then you'll find that, in a matter of hours, you will have reduced your weight by two thirds. -Weight is a vector and its direction of pull is towards the centre of the planet you're stood on. -The weight of any given object can go up or down depending on the amount of gravity acting on it. More gravity - the heavier the object.