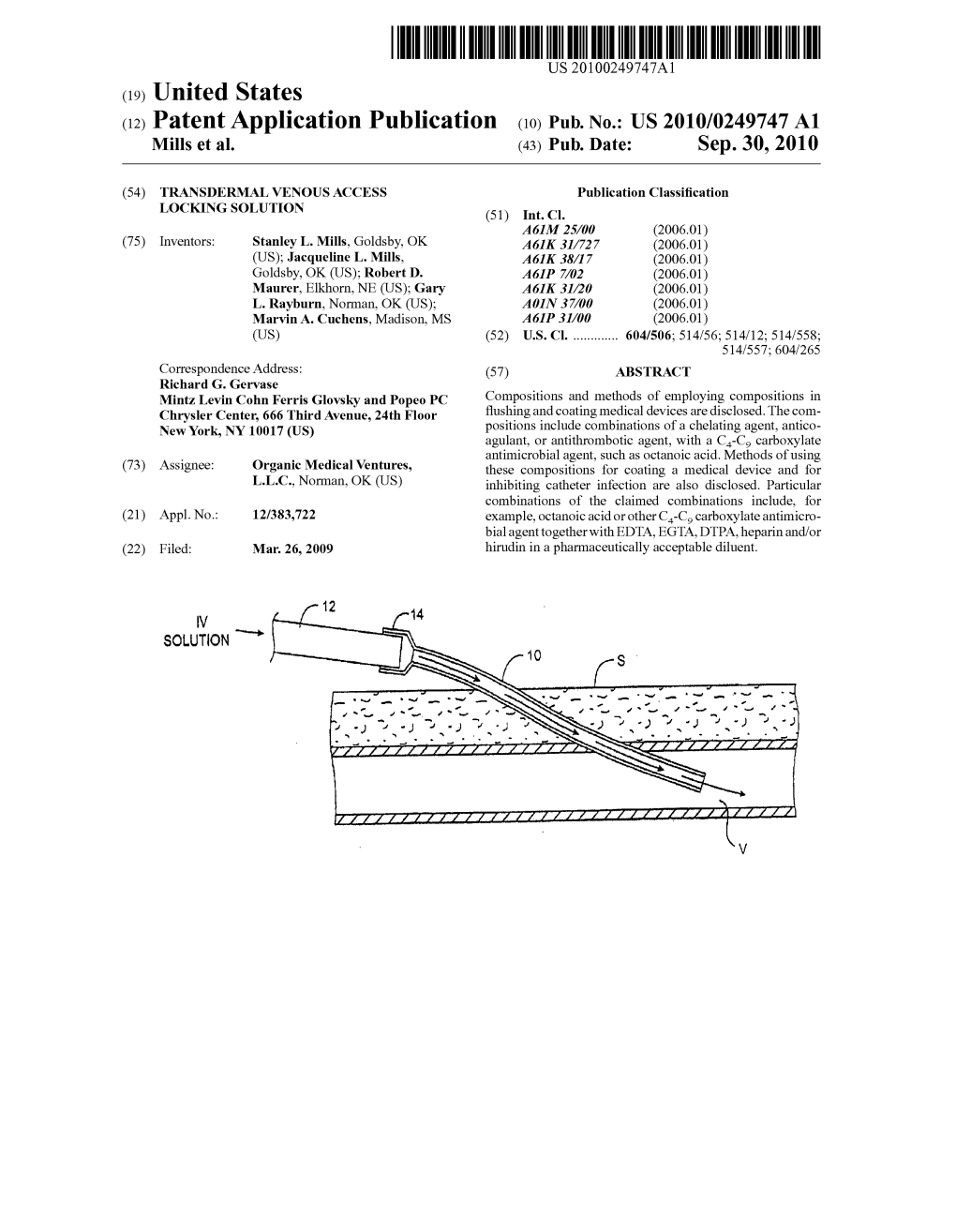
(12) Patent Application Publication (10) Pub. No.: US 2010/0249747 A1 Mills Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Table Summarizes Changes to the HF Qxq As of 10/11/2019 Question
Updated HFS Instructions (QxQs) This table summarizes changes to the HF QxQ as of 10/11/2019 Question in HF QxQ Description of Changes in HF QXQ General Instructions, pg. 3 Clarification added to rules for history Q29.d.10., pg. 40 Clarification made on how to record pulmonary hypertention Q29.d.14., pg. 41 Clarification made on how to record diastolic dysfunction Q42., pg. 42 Clarification made on how to record troponin I Section VII: Medication, pg. 54 Clarificaiton made on how to record medications Appendix A, pg. 61 Updated list of medications Edoxaban (ACOAG, Generic) Lixiana (ACOAG, Trade) Prexxartan (ARB, Trade) INSTRUCTIONS FOR COMPLETING HEART FAILURE HOSPITAL RECORD ABSTRACTION FORM HFS Version C, 10/1/2015 HFA Version D, 10/1/2015 HF QxQ, 10/11/2019 Table of Contents Page General Instructions……………………………………………………………….. 2 Specific Items………………………………………………………………………. 3 Section l: Screening for Decompensation………………………………….. 5 Section ll: History of Heart Failure…………………………………………... 10 Section lll: Medical History ………………………………………………….. 13 Section lV: Physical Exam - Vital Signs…………………………………….. 24 Section V: Physical Exam - Findings……………………………………….. 26 Section Vl: Diagnostic Tests…………………………………………………. 31 Section Vll: Biochemical Analyses………………………………………….. 48 Section Vlll: Interventions…………………………………………………….. 51 Section lX: Medications………………………………………………………. 54 Section X: Complications Following Events………………………………… 59 Section Xl: Administrative……………………………………………………. 60 Appendix A: ARIC Heart Failure/Cardiac Drugs: ………………………………. 61 Alphabetical Sort Appendix B: Potential Scenarios of the Onset of Heart………………………. 73 Failure Event or Decompensation HF QxQ 10/11/2019 Page 1 of 73 General Instructions The HFAA form was initially used for all discharges selected for HF surveillance. It was replaced by the HFAB and HFSA forms and then updated June 2012 with HFAC and HFSB. -

Feeding Acetyl-L-Carnitine and Lipoic Acid to Old Rats Significantly Improves Metabolic Function While Decreasing Oxidative Stress
Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress Tory M. Hagen*, Jiankang Liu†‡, Jens Lykkesfeldt§, Carol M. Wehr†, Russell T. Ingersoll‡, Vladimir Vinarsky†, James C. Bartholomew¶, and Bruce N. Ames†‡ʈ *Department of Biochemistry and Biophysics, Linus Pauling Institute, Oregon State University, Corvallis, OR 97331; †Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720; ‡Children’s Hospital Oakland Research Institute, Oakland, CA 94609; §Department of Pharmacology and Pathobiology, Royal Veterinary and Agricultural University, Copenhagen DK-1870, Denmark; and ¶Lawrence Berkeley National Laboratory, Berkeley, CA 94720 Contributed by Bruce N. Ames, December 28, 2001 Mitochondrial-supported bioenergetics decline and oxidative antioxidants or mitochondrial bioenergetics (13–15). Feeding stress increases during aging. To address whether the dietary old rats acetyl-L-carnitine (ALCAR), a mitochondrial metabo- addition of acetyl-L-carnitine [ALCAR, 1.5% (wt͞vol) in the drinking lite, reverses the age-related decline in tissue carnitine levels and water] and͞or (R)-␣-lipoic acid [LA, 0.5% (wt͞wt) in the chow] improves mitochondrial fatty acid -oxidation in the tissues improved these endpoints, young (2–4 mo) and old (24–28 mo) studied (15–18). ALCAR supplementation also reverses the F344 rats were supplemented for up to 1 mo before death and age-related alterations in fatty acid profiles and loss in cardio- ؉ hepatocyte isolation. ALCAR LA partially reversed the age-related lipin levels, an essential phospholipid required for mitochondrial decline in average mitochondrial membrane potential and signif- substrate transport (15–17). We demonstrated that ALCAR ؍ icantly increased (P 0.02) hepatocellular O2 consumption, indi- supplementation reverses the age-associated decline in meta- cating that mitochondrial-supported cellular metabolism was -؉ bolic activity in rats, suggesting that ALCAR improves mito markedly improved by this feeding regimen. -
MIRADON FPO Brand of Anisindione Tablets
R 1 2 3 4 5 3 4 F-16099775 1898 ® MIRADON FPO brand of anisindione Tablets DESCRIPTION MIRADON Tablets contain a syn- thetic anticoagulant, anisindione, an indanedione derivative. Each tablet contains 50 mg anisin- dione. They also contain: corn starch, FD&C Red No. 3, gelatin, lactose, and hydrogenated cotton- seed oil. ACTIONS Like phenindione, to which it is re- lated chemically, anisindione exercises its thera- peutic action by reducing the prothrombin activity of the blood. INDICATIONS Anisindione is indicated for the prophylaxis and treatment of venous thrombosis and its extension, the treatment of atrial fibrilla- tion with embolization, the prophylaxis and treat- ment of pulmonary embolism, and as an adjunct in the treatment of coronary occlusion. CONTRAINDICATIONS All contraindications to oral anticoagulant therapy are relative rather than absolute. Contraindications should be evaluated for each patient, giving consideration to the need for and the benefits to be achieved by anticoagu- lant therapy, the potential dangers of hemor- rhage, the expected duration of therapy, and the quality of patient monitoring and compliance. Hemorrhagic Tendencies or Blood Dyscrasias: In general, oral anticoagulants are contraindi- cated in patients who are bleeding or who have hemorrhagic blood dyscrasias or hemorrhagic tendencies (eg, hemophilia, polycythemia vera, purpura, leukemia) or a history of bleeding dia- thesis. They are contraindicated in patients with recent cerebral hemorrhage, active ulceration of the gastrointestinal tract, including ulcerative colitis, or open ulcerative, traumatic, or surgical wounds. Oral anticoagulants may be contraindi- cated in patients with recent or contemplated brain, eye, or spinal cord surgery or prostatec- tomy, and in those undergoing regional or lumbar block anesthesia or continuous tube drainage of the small intestine. -

Fe2+-Induced Lysis and Lipid Peroxidation of Chromaffin Granules
Journal of Neurochemisrry Raven Press, New York 0 1985 International Society for Neurochemistry Fe2’-Induced Lysis and Lipid Peroxidation of Chromaffin Granules Ronald M. Spears and Ronald W. Holz Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, Michigan, U.S.A Abstract: Chromaffin granules, the catecholaminergic by trace amounts of reducible polyvalent cation. Lysis storage granules from adrenal chromaffin cells, lysed in sometimes occurred when Ca2+ was added with EGTA 10-9-10-7M Fez+. Lysis was accompanied by the pro- (10 pLM free Ca2+ concentration) and was consistently duction of malondialdehyde which results from lipid per- observed together with malondialdehyde production in oxidation. Both chromaffin granule lysis and malondi- the presence of Ca2+,EGTA, and 10 pM Fez+(total con- aldehyde production were inhibited by the free radical centration). The apparent Ca2+ dependency for chro- trapping agent butylated hydroxytoluene but not by cat- maffin granule lysis and malondialdehyde production was alase and/or superoxide dismutase. The results suggest probably caused by a trace reducible polyvalent ion dis- that lysis resulted from a direct transfer of electrons from placed by Ca2+from EGTA and not by a Ca2+-dependent Fe2+to a component of the chromaffin granule membrane reaction involving the chromaffin granule. Key Words: without the participation of either superoxide or hy- Chrornaffin granules- Fe2+- Malondialde hyde- Lipid drogen peroxide and may have resulted from lipid per- peroxidation-Ca2+. Spears R. M. and Holz R. W. oxidation. In some experiments, ascorbate alone induced Fe2+-induced lysis and lipid peroxidation of chromaffin chromaffin granule lysis which was inhibited by EDTA, granules. -

Deferiprone and Efonidipine Mitigated Iron-Overload Induced Neurotoxicity
Life Sciences 239 (2019) 116878 Contents lists available at ScienceDirect Life Sciences journal homepage: www.elsevier.com/locate/lifescie Deferiprone and efonidipine mitigated iron-overload induced neurotoxicity T in wild-type and thalassemic mice Jirapas Sripetchwandeea,b, Juthamas Khamseekaewb, Saovaros Svastic, Somdet Srichairatanakoold, Suthat Fucharoenc, Nipon Chattipakorna,b, ∗ Siriporn C. Chattipakorna,b,e, a Neurophysiology Unit, Cardiac Electrophysiology Research and Training Center, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand b Department of Physiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand c Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University, Nakhon Pathom, 73170, Thailand d Department of Biochemistry, Faculty of Medicine, Chiang Mai University, 50200, Thailand e Department of Oral Biology and Diagnostic Sciences, Faculty of Dentistry, Chiang Mai University, Chiang Mai, 50200, Thailand ARTICLE INFO ABSTRACT Keywords: Aims: We previously demonstrated that iron-overload in non-thalassemic rats induced neurotoxicity and cog- Iron-overload nitive decline. However, the effect of iron-overload on the brain of thalassemic condition has never beenin- Oxidative stress vestigated. An iron chelator (deferiprone) provides neuroprotective effects against metal toxicity. Furthermore, a Iron chelator T-type calcium channels blocker (efonidipine) effectively attenuates cardiac dysfunction in thalassemic mice T-type calcium channel blockers with iron-overload. However, the effects of both drugs on brain of iron-overload thalassemia has notbeen Mitochondria determined. We hypothesize that iron-overload induces neurotoxicity in Thalassemic and wild-type mice, and not only deferiprone, but also efonidipine, provides neuroprotection against iron-overload condition. Main methods: Mice from both wild-type (WT) and β-thalassemic type (HT) groups were assigned to be fed with a standard-diet or high-iron diet containing 0.2% ferrocene/kg of diet (HFe) for 4 months consecutively. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -

Nitric Oxide Modulation of Interleukin-1Я-Evoked Intracellular
The Journal of Neuroscience, December 15, 2000, 20(24):8980–8986 Nitric Oxide Modulation of Interleukin-1-Evoked Intracellular Ca2؉ Release in Human Astrocytoma U-373 MG Cells and Brain Striatal Slices Antonella Meini,1 Alberto Benocci,1 Maria Frosini,1 Gianpietro Sgaragli,1 Gianpaolo Pessina,2 Carlo Aldinucci,2 Gise` le Tchuisseu Youmbi,1 and Mitri Palmi1 1Istituto di Scienze Farmacologiche and 2Istituto di Fisiologia, Universita` di Siena, 53100 Siena, Italy Intracellular Ca 2ϩ mobilization and release into mammal CSF Ca 2ϩ release induced by 2.5 but not 10 ng/ml IL-1. Ruthenium plays a fundamental role in the etiogenesis of fever induced by red (50 M) and, to a lesser extent, heparin (3 mg/ml) antagonized the proinflammatory cytokine interleukin-1 (IL-1) and other IL-1-induced Ca 2ϩ release, and both compounds administered pyrogens. The source and mechanism of IL-1-induced intracel- together completely abolished this response. Similar results were lular Ca 2ϩ mobilization was investigated using two experimental obtained in human astrocytoma cells in which IL-1 elicited a models. IL-1 (10 ng/ml) treatment of rat striatal slices preloaded delayed (30 min) increase in intracellular Ca 2ϩ concentration 45 2ϩ 2ϩ Ϯ with Ca elicited a delayed (30 min) and sustained increase ([Ca ]i ) (402 71.2% of baseline), which was abolished by 1 45 2ϩ (125–150%) in spontaneous Ca release that was potentiated mML-NAME. These data indicate that the NO/cGMP-signaling by L-arginine (300 M) and counteracted by N--nitro-L-arginine pathway is part of the intracellular mechanism transducing IL- 2ϩ methyl ester (L-NAME) (1 and 3 mM). -

Some Aspects of the Pharmacology of Oral Anticoagulants
Some aspects of the pharmacology of oral anticoagulants The pharmacology of oral anticoagulants ls discussed with particular rejerence to data of value in the management of therapy. The importance of individual variability in response and drug interaction is stressed. Other effects of these agents which may have clinical utility are noted. William W. Coon, M.D., and Park W. Willis 111, M.D., Ann Arbor, Mich. The Departments of Surgery (Section of General Surgery) and Medicine, University of Michigan Medical School In the twenty-five years sinee the isola dividual struetural features but by a com tion of the hemorrhagie faetor in spoiled bination of several: molecular shape, in sweet clever," the gradually inereasing creased aetivity with 6 membered hetero utilization of oral antieoagulants for the eyclic rings with a substituent in position prevention and therapy of thromboembolie 8 and with a methoxyl rather than a free disease has made them one of the most hydroxl group. Also important is the dernon widely used groups of pharmacologic stration that levorotatory warfarin is seven agents. This review is restrieted to as times more aetive than its enantiomer.F" peets of the pharmaeology of these agents As Hunter and Shepherd'" have pointed whieh may be important to their proper out, the failure to obtain a precise cor clinieal utilization. relation between strueture and antieoagu lant aetivity is "not surprising in view of Relation of structure to function the influence of small struetural changes The oral antieoagulants have been di on sueh variables as solubility, rate of ab vided into four main groups on the basis sorption, ease of distribution, degree of of ehemieal strueture (Fig. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0271461 A1 Reb Et Al
US 20140271461A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0271461 A1 Reb et al. (43) Pub. Date: Sep. 18, 2014 (54) COMPOSITIONS AND ASSOCATED Publication Classification METHODS FOR RADIOSOTOPE-BINDING MCROPARTICLES (51) Int. Cl. A615 L/2 (2006.01) (71) Applicant: Biosphere Medical, Inc., South Jordan, (52) U.S. Cl. UT (US) CPC .................................. A61K 51/1244 (2013.01) USPC ........................... 424/1.37: 525/360; 428/402 (72) Inventors: Philippe Reb, Themericourt (FR); Celine Chaix, Clichy la Garenne (FR) (57) ABSTRACT (73) Assignee: Biosphere Medical, Inc., South Jordan, The present disclosure relates to polymeric materials that UT (US) may be labeled with a radioisotope, to processes for produc ing the labeled polymeric material, and to methods of using (21) Appl. No.: 14/207,219 the materials in analytical and therapeutic applications. Spe cifically, the disclosure relates to injectable and implantable (22) Filed: Mar 12, 2014 microparticles. Such as microspheres, which are associated with radioisotopes such that the microparticles are both thera Related U.S. Application Data peutic and detectable. The radioisotope-containing micropar (60) Provisional application No. 61/779,712, filed on Mar. ticles are useful for embolization and other therapeutic medi 13, 2013. cal applications. Patent Application Publication Sep. 18, 2014 Sheet 1 of 11 US 2014/0271461 A1 FIG. 1 Patent Application Publication Sep. 18, 2014 Sheet 2 of 11 US 2014/0271461 A1 FIG. 2 Patent Application Publication Sep. 18, 2014 Sheet 3 of 11 US 2014/0271461 A1 FIG 3 Patent Application Publication Sep. 18, 2014 Sheet 4 of 11 US 2014/0271461 A1 FIG. -

Home Medication Form
DIVISION OF EDUCATION • SURGICAL PATIENT EDUCATION Medication and Surgery Inspiring Quality: BEFORE YOUR OPERATION Highest Standards, Better Outcomes Your medications may have to be adjusted before your operation. Some medication can affect your recovery and response to anesthesia. Write down all of the mediations you are taking. A blank medication list is provided if you need it. Make a list. Your list should include: ● Any prescription medications ● Over-the-counter (OTC) medications (such as aspirin or Tylenol) ● Herbs, vitamins, and supplements ● Tell your doctor if you smoke and how often you drink alcohol or use other recreational drugs. Check with your List of medications that affect blood clotting:* doctor about: ● Antiplatelet Medication: Anagrelide (Agrylin®), aspirin (any brand, all doses), cilostazol (Pletal®), clopidogrel (Plavix®), ● When to stop taking all vitamins, dipyradamole (Persantine®), dipyridamole/aspirin (Aggrenox®), herbs, and diet supplements. enteric-coated aspirin (Ecotrin®), ticlopidine (Ticlid®) This could be 10 to 14 days ● ® before and up to 7 days after Anticoagulant Medication: Anisindione (Miradon ), Arixtra, ® your test or operation. enoxaparin (Lovenox ) injection, Fragmin, heparin injection, Pradaxa, pentosan polysulfate (Elmiron®), warfarin (Coumadin®), Xerelto ● How to take your medication on ● the morning of your operation. You Nonsteroidal Anti-Inflammatory Drugs: Celebrex, diclofenac ® ® ® ® may be instructed to take some (Voltaren , Cataflam ), diflunisal (Dolobid ), etodolac (Lodine ), fenoprofen -

Appendix C Medication Tables
Appendix C Medication Tables Note: The medication tables are not meant to be inclusive lists of all available therapeutic agents. Approved medication tables will be updated regularly. Discrepancies must be reported. See Resource Section of this manual for additional contact information. Release Notes: Aspirin Table Version 1.0 Table 1.1 Aspirin and Aspirin-Containing Medications Acetylsalicylic Acid Acuprin 81 Alka-Seltzer Alka-Seltzer Morning Relief Anacin Arthritis Foundation Aspirin Arthritis Pain Ascriptin Arthritis Pain Formula ASA ASA Baby ASA Baby Chewable ASA Baby Coated ASA Bayer ASA Bayer Children's ASA Buffered ASA Children's ASA EC ASA Enteric Coated ASA/Maalox Ascriptin Aspergum Aspir-10 Aspir-Low Aspir-Lox Aspir-Mox Aspir-Trin Aspirbuf Aspircaf Aspirin Aspirin Baby Aspirin Bayer Aspirin Bayer Children's Aspirin Buffered Aspirin Child Aspirin Child Chewable Aspirin Children's Aspirin EC Aspirin Enteric Coated Specifications Manual for National Appendix C-1 Hospital Quality Measures Table 1.1 Aspirin and Aspirin-Containing Medications (continued) Aspirin Litecoat Aspirin Lo-Dose Aspirin Low Strength Aspirin Tri-Buffered Aspirin, Extended Release Aspirin/butalbital/caffeine Aspirin/caffeine Aspirin/pravachol Aspirin/pravastatin Aspirtab Bayer Aspirin Bayer Aspirin PM Extra Strength Bayer Children’s Bayer EC Bayer Enteric Coated Bayer Low Strength Bayer Plus Buffered ASA Buffered Aspirin Buffered Baby ASA Bufferin Bufferin Arthritis Strength Bufferin Extra Strength Buffex Cama Arthritis Reliever Child’s Aspirin Coated Aspirin -

I ©Copyright 2012 Clara K. Hsia
©Copyright 2012 Clara K. Hsia i Biochemical and Mechanistic Studies of the Interactions Between Vitamin K Antagonists and Vitamin K Epoxide Reductase Clara K. Hsia A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2012 Reading Committee: Allan E. Rettie, Chair Kent L. Kunze William M. Atkins Program Authorized to Offer Degree: Medicinal Chemistry University of Washington Abstract Biochemical and Mechanistic Studies of the Interactions Between Vitamin K Antagonists and Vitamin K Epoxide Reductase Clara K. Hsia Chair of the Supervisory Committee: Professor Allan E. Rettie Department of Medicinal Chemistry The studies that are presented in this dissertation have; (i) established a mechanism by which warfarin dose response is modulated by VKORC1 genotype under optimized conditions for kinetic analysis of human vitamin K epoxide reductase (VKOR) catalytic activity, (ii) evaluated previously suggested irreversible and reversible mechanisms of VKOR inhibition by vitamin K antagonists (VKAs), and (iii) elucidated the role of tyrosine 139 in the catalytic center and inhibitor binding site of VKOR. Firstly, observation of VKOR activity in human liver microsomes (HLMs) under varied experimental conditions demonstrated that human VKOR is a highly labile enzyme. Under optimal conditions, evaluation of genotyped HLMs for downstream effects of altered VKORC1 mRNA expression revealed a relationship between the VKORC1 B haplotype and increased the catalytic activity and protein expression of VKOR. This demonstrates that warfarin dose is regulated through a transcriptional mechanism wherein higher levels of VKORC1 mRNA translate to increased protein expression and associated enzymatic activity of VKOR, thus requiring a higher dose of warfarin to maintain the same target level of anticoagulation.