The Importance of Blueschist —» Eclogite Dehydration Reactions in Subducting Oceanic Crust
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Polyphase Deformation in San Miguel Las Minas, Northern
POLYPHASE DEFORMATION IN SAN MIGUEL LAS MINAS, NORTHERN ACATLAN COMPLEX, SOUTHERN MEXICO A thesis presented to the faculty of the Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Master of Sciences Brent J. Barley August 2006 This thesis entitled POLYPHASE DEFORMATION IN SAN MIGUEL LAS MINAS, NORTHERN ACATLAN COMPLEX, SOUTHERN MEXICO by BRENT J. BARLEY has been approved for the Department of Geological Sciences and the College of Arts and Sciences by R. Damian Nance Professor of Geological Sciences Benjamin M. Ogles Dean, College of Arts and Sciences Abstract BARLEY, BRENT J., M.S., August 2006, Geological Sciences POLYPHASE DEFORMATION IN SAN MIGUEL LAS MINAS, NORTHERN ACATLAN COMPLEX, SOUTHERN MEXICO (58 pages) Director of Thesis: R. Damian Nance Mapping in the northern part of the Acatlán Complex (southern Mexico) has distinguished two lithological units: a high-grade unit assigned to the Piaxtla Suite, and a low-grade unit assigned to the Cosoltepec Formation. Two major Paleozoic tectonothermal events have been identified in these rocks. The first event produced a penetrative deformational fabric (SPS1) parallel to a compositional banding during blueschist and amphibolite facies metamorphism, which has recently been dated as ~346 Ma in a neighboring area, and a greenschist overprint during exhumation. The second event, which is recorded in both the Piaxtla Suite and Cosoltepec Formation, produced two penetrative deformational fabrics under subgreenschist metamorphic conditions. The first, high-grade tectonothermal event accompanied closure of the Rheic Ocean and tectonic juxtapositioning of the two units during exhumation of the high-grade unit in the Devono-Carboniferous. -

Facies and Mafic
Metamorphic Facies and Metamorphosed Mafic Rocks l V.M. Goldschmidt (1911, 1912a), contact Metamorphic Facies and metamorphosed pelitic, calcareous, and Metamorphosed Mafic Rocks psammitic hornfelses in the Oslo region l Relatively simple mineral assemblages Reading: Winter Chapter 25. (< 6 major minerals) in the inner zones of the aureoles around granitoid intrusives l Equilibrium mineral assemblage related to Xbulk Metamorphic Facies Metamorphic Facies l Pentii Eskola (1914, 1915) Orijärvi, S. l Certain mineral pairs (e.g. anorthite + hypersthene) Finland were consistently present in rocks of appropriate l Rocks with K-feldspar + cordierite at Oslo composition, whereas the compositionally contained the compositionally equivalent pair equivalent pair (diopside + andalusite) was not biotite + muscovite at Orijärvi l If two alternative assemblages are X-equivalent, l Eskola: difference must reflect differing we must be able to relate them by a reaction physical conditions l In this case the reaction is simple: l Finnish rocks (more hydrous and lower MgSiO3 + CaAl2Si2O8 = CaMgSi2O6 + Al2SiO5 volume assemblage) equilibrated at lower En An Di Als temperatures and higher pressures than the Norwegian ones Metamorphic Facies Metamorphic Facies Oslo: Ksp + Cord l Eskola (1915) developed the concept of Orijärvi: Bi + Mu metamorphic facies: Reaction: “In any rock or metamorphic formation which has 2 KMg3AlSi 3O10(OH)2 + 6 KAl2AlSi 3O10(OH)2 + 15 SiO2 arrived at a chemical equilibrium through Bt Ms Qtz metamorphism at constant temperature and = -

Geology the Geotrail Follows Rocks Exposed on the Beaches South of Port Macquarie
Geology The Geotrail follows rocks exposed on the beaches south of Port Macquarie. These rocks record a fascinating story involving the migration of an oceanic plate away from a mid-ocean ridge (oceanic spreading ridge) to a subduction zone about 500 million years ago (Figures 1, 2). Back then, our continent was part of a supercontinent called Gondwana which was located near the Equator (Figure 3). Since then, this supercontinent has migrated and broken up, with the Australian continent eventually reaching its current position (Figure 2S). To imagine this process of breaking up and migration, think of the way ice sheets in Antarctica crack and float across the ocean carried by ocean currents. Figure 1 shows the migration of oceanic crust away from a mid-ocean ridge exuding basalt (mid ocean ridge basalt - MORB; Shelly Beach) and down the subduction zone (Rocky Beach). Figure 2 Geological Time Scale Walking the geotrail allows you to track the migration of tectonic plates, observe how the rocks change, and learn about the setting in which they formed. At Shelly Beach (Stop 1), are dark rocks called basalt that are thought to have formed close to a spreading ridge (the boundary between two divergent tectonic plates; Figures 1S, 4) because their chemical composition is similar to mid-oceanic ridge basalts (Och 2007). The Mid-Atlantic Ridge that divides the North American plate from the African plate is an example of this type of plate border (Figure 4). Figure 3 shows the supercontinent Gondwana and the Australian continent as part of Gondwana. The Australian continent was at the Equator at this time. -

Petrologic and Textural Examination of Blueschist-Facies Micaceous Schists of Syros, Greece
PETROLOGIC AND TEXTURAL EXAMINATION OF BLUESCHIST-FACIES MICACEOUS SCHISTS OF SYROS, GREECE. Joshua W. Otis Dept. Of Geology, Amherst College, Amherst, MA, 01002 Faculty Sponsors: John T. Cheney, Tekla Harms, Amherst College INTRODUCTION The island of Syros lies in the high pressure belt of the Attico -Cycladic crystalline massif. It is composed dominantly of metasedimentary and meta-igneous rocks with local areas of melange and serpentinite zones. These rocks preserve blueschist facies mineral assemblages, although an incomplete greenschist overprint exists locally across the island. This study focuses on the semi-pelitic and calcareous schists of Syros. The schist units are interlayered with marble across the island, and are laterally continuous parallel to the strike of the foliation, with beds generally dipping 25-45° to the NE (Hecht, 1985). The schists from the north end of the island were very consistent, laterally continuous units. Over the rest of the island, the schists tended to be much more locally variable in composition, Ideally, this project aims to integrate structural, petrographic, and compositional data to characterize the nature and timing of deformation relative to mineral growth, and to place constraints on the P/T conditions experienced by semi-pelitic rocks across the island. PETROGRAPHY The north end of the island contains calcareous schists with the relatively uniform mineral assemblages of quartz + phengite ± calcite ± minor amounts of sodic amphibole, garnet, epidote, rutile, titanite, and graphite. These rocks are divisible into two main groups based on the presence or absence of calcite, forming two fairly homogenous, mappable units (~1km scale). A greenschist overprint of albite + chlorite +/- epidote typically occurs in these rocks (Fig. -

The Origin of Formation of the Amphibolite- Granulite Transition
The Origin of Formation of the Amphibolite- Granulite Transition Facies by Gregory o. Carpenter Advisor: Dr. M. Barton May 28, 1987 Table of Contents Page ABSTRACT . 1 QUESTION OF THE TRANSITION FACIES ORIGIN • . 2 DEFINING THE FACIES INVOLVED • • • • • • • • • • 3 Amphibolite Facies • • • • • • • • • • 3 Granulite Facies • • • • • • • • • • • 5 Amphibolite-Granulite Transition Facies 6 CONDITIONS OF FORMATION FOR THE FACIES INVOLVED • • • • • • • • • • • • • • • • • 8 Amphibolite Facies • • • • • • • • 9 Granulite Facies • • • • • • • • • •• 11 Amphibolite-Granulite Transition Facies •• 13 ACTIVITIES OF C02 AND H20 . • • • • • • 1 7 HYPOTHESES OF FORMATION • • • • • • • • •• 19 Deep Crust Model • • • • • • • • • 20 Orogeny Model • • • • • • • • • • • • • 20 The Earth • • • • • • • • • • • • 20 Plate Tectonics ••••••••••• 21 Orogenic Events ••••••••• 22 Continent-Continent Collision •••• 22 Continent-Ocean Collision • • • • 24 CONCLUSION • . • 24 BIBLIOGRAPHY • • . • • • • 26 List of Illustrations Figure 1. Precambrian shields, platform sediments and Phanerozoic fold mountain belts 2. Metamorphic facies placement 3. Temperature and pressure conditions for metamorphic facies 4. Temperature and pressure conditions for metamorphic facies 5. Transformation processes with depth 6. Temperature versus depth of a descending continental plate 7. Cross-section of the earth 8. Cross-section of the earth 9. Collision zones 10. Convection currents Table 1. Metamorphic facies ABSTRACT The origin of formation of the amphibolite granulite transition -

Cold Subduction and the Formation of Lawsonite Eclogite – Constraints from Prograde Evolution of Eclogitized Pillow Lava from Corsica
J. metamorphic Geol., 2010, 28, 381–395 doi:10.1111/j.1525-1314.2010.00870.x Cold subduction and the formation of lawsonite eclogite – constraints from prograde evolution of eclogitized pillow lava from Corsica E. J. K. RAVNA,1 T. B. ANDERSEN,2 L. JOLIVET3 AND C. DE CAPITANI4 1Department of Geology, University of Tromsø, N-9037 Tromsø, Norway ([email protected]) 2Department of Geosciences and PGP, University of Oslo, PO Box 1047, Blindern, 0316 Oslo, Norway 3ISTO, UMR 6113, Universite´ dÕOrle´ans, 1A Rue de la Fe´rollerie, 45071 Orle´ans, Cedex 2, France 4Mineralogisch-Petrographisches Institut, Universita¨t Basel, Bernoullistrasse 30, 4056 Basel, Switzerland ABSTRACT A new discovery of lawsonite eclogite is presented from the Lancoˆne glaucophanites within the Schistes Lustre´ s nappe at De´ file´ du Lancoˆne in Alpine Corsica. The fine-grained eclogitized pillow lava and inter- pillow matrix are extremely fresh, showing very little evidence of retrograde alteration. Peak assemblages in both the massive pillows and weakly foliated inter-pillow matrix consist of zoned idiomorphic Mg-poor (<0.8 wt% MgO) garnet + omphacite + lawsonite + chlorite + titanite. A local overprint by the lower grade assemblage glaucophane + albite with partial resorption of omphacite and garnet is locally observed. Garnet porphyroblasts in the massive pillows are Mn rich, and show a regular prograde growth-type zoning with a Mn-rich core. In the inter-pillow matrix garnet is less manganiferous, and shows a mutual variation in Ca and Fe with Fe enrichment toward the rim. Some garnet from this rock type shows complex zoning patterns indicating a coalescence of several smaller crystallites. -

Geology of the Prince William Sound and Kenai Peninsula Region, Alaska
Geology of the Prince William Sound and Kenai Peninsula Region, Alaska Including the Kenai, Seldovia, Seward, Blying Sound, Cordova, and Middleton Island 1:250,000-scale quadrangles By Frederic H. Wilson and Chad P. Hults Pamphlet to accompany Scientific Investigations Map 3110 View looking east down Harriman Fiord at Serpentine Glacier and Mount Gilbert. (photograph by M.L. Miller) 2012 U.S. Department of the Interior U.S. Geological Survey Contents Abstract ..........................................................................................................................................................1 Introduction ....................................................................................................................................................1 Geographic, Physiographic, and Geologic Framework ..........................................................................1 Description of Map Units .............................................................................................................................3 Unconsolidated deposits ....................................................................................................................3 Surficial deposits ........................................................................................................................3 Rock Units West of the Border Ranges Fault System ....................................................................5 Bedded rocks ...............................................................................................................................5 -

A Systematic Nomenclature for Metamorphic Rocks
A systematic nomenclature for metamorphic rocks: 1. HOW TO NAME A METAMORPHIC ROCK Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: Web version 1/4/04. Rolf Schmid1, Douglas Fettes2, Ben Harte3, Eleutheria Davis4, Jacqueline Desmons5, Hans- Joachim Meyer-Marsilius† and Jaakko Siivola6 1 Institut für Mineralogie und Petrographie, ETH-Centre, CH-8092, Zürich, Switzerland, [email protected] 2 British Geological Survey, Murchison House, West Mains Road, Edinburgh, United Kingdom, [email protected] 3 Grant Institute of Geology, Edinburgh, United Kingdom, [email protected] 4 Patission 339A, 11144 Athens, Greece 5 3, rue de Houdemont 54500, Vandoeuvre-lès-Nancy, France, [email protected] 6 Tasakalliontie 12c, 02760 Espoo, Finland ABSTRACT The usage of some common terms in metamorphic petrology has developed differently in different countries and a range of specialised rock names have been applied locally. The Subcommission on the Systematics of Metamorphic Rocks (SCMR) aims to provide systematic schemes for terminology and rock definitions that are widely acceptable and suitable for international use. This first paper explains the basic classification scheme for common metamorphic rocks proposed by the SCMR, and lays out the general principles which were used by the SCMR when defining terms for metamorphic rocks, their features, conditions of formation and processes. Subsequent papers discuss and present more detailed terminology for particular metamorphic rock groups and processes. The SCMR recognises the very wide usage of some rock names (for example, amphibolite, marble, hornfels) and the existence of many name sets related to specific types of metamorphism (for example, high P/T rocks, migmatites, impactites). -
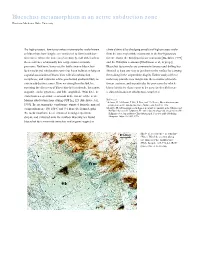
Blueschist Metamorphism in an Active Subduction Zone Hirokazu Maekawa, Kobe University
Blueschist metamorphism in an active subduction zone Hirokazu Maekawa, Kobe University The high-pressure, low-temperature metamorphic rocks known clasts obtained by dredging samples of high pressure rocks as blueschists have long been considered to form in subduc- from the two serpentinite seamounts in the Izu-Ogasawara tion zones, where the descent of a relatively cold slab leads to forearc, that is the Torishima forearc seamount [Maekawa, 1995] the occurrence of unusually low temperatures at mantle and the Hahajima seamount [Hashimoto et al., in prep.]. pressures. Until now, however, the link between blueschist- Blueschist facies rocks are common in forearcs and drilling has facies rocks and subduction zones has been indirect, relying on showed at least one way to get them to the surface by carrying a spatial association of blueschists with old subduction them along in the serpentinite diapirs. Further study of these complexes, and estimates of the geothermal gradients likely to rocks may provide new insight into the tectonics of trench- exist in subduction zones. Here we strengthen this link, by forearc systems, and in particular, the processes by which reporting the discovery of blueschist-facies minerals, lawsonite, blueschist facies clasts come to be associated with forearc aragonite, sodic pyroxene, and blue amphibole (winchite), in sediments in ancient subduction complexes. clasts from a serpentinite seamount in the forearc of the active Mariana subduction zone during ODP Leg 125 [Maekawa et al., References: Maekawa, H., M. Shozui, T. Ishii, P. Fryer, and J.A. Pearce, Blueschist metamor- 1993]. The metamorphic conditions estimated from the mineral phism in an active subduction zone, Nature, 364, 520-523, 1993. -

Chapter 5. the Eclogite Engine
Chapter 5 The eclogite engine The World's great age begins anew, being recognized that large blocks of eclogite in The golden years return, the mantle may be an important fertility source. Delaminated continental crust differs in many The Earth doth like a snake renew important respects from recycled MORB. It does Her winter weeds outgrown not go through subduction zone and seafloor pro Shelley cessing, it starts out hotter than MORB, it may occur in bigger blobs, and it is not accompanied by the same amount, if any, of buoyant infer The water cycle drives geological processes at tile harzburgite. Figure 5.1 illustrates the lower the surface. TI1e fact that water coexists as fluid, crust delamination cycle. The crust thickens by vapor and solid is crucial in shaping the Earth's tectonic or igneous processes, eventually forming surface. The fact that conditions in the upper dense eclogite that detaches and sinks into the mantle can readily convert eclogite to magma to mantle. It reaches a level of neutral buoyancy and basalt, and back, with enormous density changes, starts to warm up. Eventually it rises and forms a is crucial in global magmatism and tectonics. warm fertile patch in the mantle. If the overlying Phase changes in the mafic components of the continents have moved off, a midplate magmatic upper mantle are larger than thermal expansion province is the result. effects and they drive the eclogite engine. Thermal expansion is the main source of There are several ways to generate massive buoyancy in thermal convection of simple flu melting in the mantle; one is to bring hot mate ids. -

What We Know About Subduction Zones from the Metamorphic Rock Record
What we know about subduction zones from the metamorphic rock record Sarah Penniston-Dorland University of Maryland Subduction zones are complex We can learn a lot about processes occurring within active subduction zones by analysis of metamorphic rocks exhumed from ancient subduction zones Accreonary prism • Rocks are exhumed from a wide range of different parts of subduction zones. • Exhumed rocks from fossil subduction zones tell us about materials, conditions and processes within subduction zones • They provide complementary information to observations from active subduction systems Tatsumi, 2005 The subduction interface is more complex than we usually draw Mélange (Bebout, and Penniston-Dorland, 2015) Information from exhumed metamorphic rocks 1. Thermal structure The minerals in exhumed rocks of the subducted slab provide information about the thermal structure of subduction zones. 2. Fluids Metamorphism generates fluids. Fossil subduction zones preserve records of fluid-related processes. 3. Rheology and deformation Rocks from fossil subduction zones record deformation histories and provide information about the nature of the interface and the physical properties of rocks at the interface. 4. Geochemical cycling Metamorphism of the subducting slab plays a key role in the cycling of various elements through subduction zones. Thermal structure Equilibrium Thermodynamics provides the basis for estimating P-T conditions using mineral assemblages and compositions Systems act to minimize Gibbs Free Energy (chemical potential energy) Metamorphic facies and tectonic environment SubduconSubducon zone metamorphism zone metamorphism Regional metamorphism during collision Mid-ocean ridge metamorphism Contact metamorphism around plutons Determining P-T conditions from metamorphic rocks Assumption of chemical equilibrium Classic thermobarometry Based on equilibrium reactions for minerals in rocks, uses the compositions of those minerals and their thermodynamic properties e.g. -

Metamorphic Facies Metamorphic Grade Metamorphic Zones
Metamorphic Grade • Controlled by the temperature of Metamorphic Facies metamorphism • Low-grade rocks contain hydrated and Best, Chapter 10 carbonated phases • High-grade rocks are dehydrated and decarbonized Metamorphic Zones • Mappable metamorphic units of similar grade in a rock of distinct composition • Isograds are lines marking the first appearance of key minerals • Zones of Barrow – Biotite, garnet, staurolite, kyanite, sillimanite Metamorphic Reactions Metamorphic Facies • Formation of talc in a siliceous dolomite Dol + Qtz + H20 = Tlc + Cal + C02 • The set of mineral assemblages occurring in • Controlled by the ratio of H 0/ C0 2 2 rocks of diverse composition • Facies develop under restricted P,T conditions • Mineral assemblages may be plotted on ACF diagrams Main Facies Review of Facies • Zeolite • Prehnite- • Mineral assemblages in metamorphosed pumpellyite mafic rocks •Blueschist • Greenschist • Correlation of Barrow’s zones with facies • Amphibolite from different protoliths • Granulite • P, T diagram for various facies • Eclogite P,T Relations Review of Phase Diagrams Jadeite + Quartz = Albite • Solid-solid reactions • Governed by Clapeyron equation – dP/dT = 10 ∆H/T ∆V = ∆S/∆V – ∆H is the heat of reaction – ∆S is the change in entropy – ∆V is the change in volume • The slope of the stability is dP/dT Open System Models - H2O Open System Models - CO2 • Dehydration curves • Dehydration curves • Example of the general • Example of the general case case • Specific minerals • Specific minerals – Breakdown of chlorite, – Breakdown of calcite, muscovite, biotite, etc dolomite, etc Univariant Curves • Curves that define reactions with one degree of freedom • In P-T space this means that if T is changed, than P must also change to maintain equilibrium • Many important metamorphic reactions are defined by these curves P-T Examples Stability of Iron Oxides • PO2 vs.