Effects of Nitrogen Availability and Climate on Hermes Copper Butterfly
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
![Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167]](https://docslib.b-cdn.net/cover/5635/docket-no-fws-hq-es-2019-0009-ff09e21000-fxes11190900000-167-75635.webp)
Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167]
This document is scheduled to be published in the Federal Register on 10/10/2019 and available online at https://federalregister.gov/d/2019-21478, and on govinfo.gov DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 17 [Docket No. FWS–HQ–ES–2019–0009; FF09E21000 FXES11190900000 167] Endangered and Threatened Wildlife and Plants; Review of Domestic and Foreign Species That Are Candidates for Listing as Endangered or Threatened; Annual Notification of Findings on Resubmitted Petitions; Annual Description of Progress on Listing Actions AGENCY: Fish and Wildlife Service, Interior. ACTION: Notice of review. SUMMARY: In this candidate notice of review (CNOR), we, the U.S. Fish and Wildlife Service (Service), present an updated list of plant and animal species that we regard as candidates for or have proposed for addition to the Lists of Endangered and Threatened Wildlife and Plants under the Endangered Species Act of 1973, as amended. Identification of candidate species can assist environmental planning efforts by providing advance notice of potential listings, and by allowing landowners and resource managers to alleviate threats and thereby possibly remove the need to list species as endangered or threatened. Even if we subsequently list a candidate species, the early notice provided here could result in more options for species management and recovery by prompting earlier candidate conservation measures to alleviate threats to the species. This document also includes our findings on resubmitted petitions and describes our 1 progress in revising the Lists of Endangered and Threatened Wildlife and Plants (Lists) during the period October 1, 2016, through September 30, 2018. -
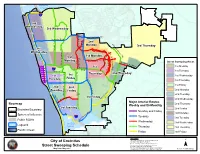
Streetsweepingmap.Pdf
Batiquitos ! ! Lagoon ! ! ! ! ! La Costa Ave ! ! ! ! ! ! 5 ! ! ¨¦§ ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 2nd ! ! ! ! ! Wednesday ! 3rd Wednesday ! ! ! G 101 ! a ¤£ ! r ! d e li ve nh ain R d ! O ! n vd ! ! Le uc ad ia B l V ! ! i d e 2nd w R N N y R R E n d Monday a l n 3rd Thursday o c r C x h o a D 1st a G l S S m en A a s rb o n i r t n D a Wednesday n r N e F o e d R r R y a W d a 1st Monday e W k i a G a r l 3rd i r l r l o a D l b a D i t r w P a is r e i V a u nt ain Monday s t M o o E V e l Street Sweeping Areas u p C n b a g m r i r n Q a o a i D l A e n l C t l No C S i r W g te D 1st Monday n Enc 1st V in it as e r 5 B l § lv d G R¨¦eq ue za S t r 1st Tuesday d D R r 2nd Thursday Thursday u o 1st 1st Wednesday o 1st d l r S a a Friday Tuesday B N E W K S t l 1st Thursday San ta F e D r C a m 1st Friday n i n 2nd o o n 2nd R e n i v e 2nd Monday Tuesday k a A Friday e r c e r k l t 101 a s a ¤£ D e S M L h 2nd Tuesday a c e n m D n v h a r 3rd Friday a A E in g M l r m ij i 2nd Wednesday o B A v Major Arterial Routes Basemap e 2nd Thursday Weekly and Bi-Monthly 3rd Tuesday Encinitas Boundary 2nd Friday ! ! ! v e Monday and Friday A r 3rd Monday te ! ! ! Sphere of Influence s e 5 h Tuesday ¨¦§ c n 3rd Tuesday a Public ROWs M Wednesday San Elijo 3rd Wednesday Lagoons Lagoon Thursday 3rd Thursday Pacific Ocean Friday 3rd Friday DISCLAIMER: This map should not be used for Engineering, City of Encinitas Survey, or Site-Specific Analysis. -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -

Guidelines for Determining Significance and Report Format and Content Requirements
COUNTY OF SAN DIEGO GUIDELINES FOR DETERMINING SIGNIFICANCE AND REPORT FORMAT AND CONTENT REQUIREMENTS BIOLOGICAL RESOURCES LAND USE AND ENVIRONMENT GROUP Department of Planning and Land Use Department of Public Works Fourth Revision September 15, 2010 APPROVAL I hereby certify that these Guidelines for Determining Significance for Biological Resources, Report Format and Content Requirements for Biological Resources, and Report Format and Content Requirements for Resource Management Plans are a part of the County of San Diego, Land Use and Environment Group's Guidelines for Determining Significance and Technical Report Format and Content Requirements and were considered by the Director of Planning and Land Use, in coordination with the Director of Public Works on September 15, 2O1O. ERIC GIBSON Director of Planning and Land Use SNYDER I hereby certify that these Guidelines for Determining Significance for Biological Resources, Report Format and Content Requirements for Biological Resources, and Report Format and Content Requirements for Resource Management Plans are a part of the County of San Diego, Land Use and Environment Group's Guidelines for Determining Significance and Technical Report Format and Content Requirements and have hereby been approved by the Deputy Chief Administrative Officer (DCAO) of the Land Use and Environment Group on the fifteenth day of September, 2010. The Director of Planning and Land Use is authorized to approve revisions to these Guidelines for Determining Significance for Biological Resources and Report Format and Content Requirements for Biological Resources and Resource Management Plans except any revisions to the Guidelines for Determining Significance presented in Section 4.0 must be approved by the Deputy CAO. -

Rin 1018–Bc57
This document is scheduled to be published in the Federal Register on 01/08/2020 and available online at https://federalregister.gov/d/2019-28461, and on govinfo.gov DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 17 [Docket No. FWS–R8–ES–2017–0053; 4500030113] RIN 1018–BC57 Endangered and Threatened Wildlife and Plants; Threatened Species Status for the Hermes Copper Butterfly with 4(d) Rule and Designation of Critical Habitat AGENCY: Fish and Wildlife Service, Interior. ACTION: Proposed rule. SUMMARY: We, the U.S. Fish and Wildlife Service (Service), propose to list the Hermes copper butterfly (Lycaena [Hermelycaena] hermes), a butterfly species from San Diego County, California, and Baja California, Mexico, as a threatened species and propose to designate critical habitat for the species under the Endangered Species Act (Act). If we finalize this rule as proposed, it would extend the Act’s protections to this species as described in the proposed rule provisions issued under section 4(d) of the Act, and designate approximately 14,249 hectares (35,211 acres) of critical habitat in San Diego County, California. We also announce the availability of a draft economic analysis (DEA) of the proposed designation of critical habitat for the Hermes copper butterfly. DATES: We will accept comments received or postmarked on or before [INSERT DATE 60 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. Comments submitted electronically using the Federal eRulemaking Portal (see ADDRESSES below) must be received by 11:59 p.m. Eastern Time on the closing date. We must receive requests for public hearings, in writing, at the address shown in FOR FURTHER INFORMATION CONTACT by [INSERT DATE 45 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER]. -

New Data on Little Known and Rare Species Athamanthia Japhetica (Nekrutenko Et Effendi, 1983) (Lepidoptera, Lycaenidae) from the Caucasus
Евразиатский энтомол. журнал 18(3): 213–216 © EUROASIAN ENTOMOLOGICAL doi: 10.15298/euroasentj.18.3.12 JOURNAL, 2019 New data on little known and rare species Athamanthia japhetica (Nekrutenko et Effendi, 1983) (Lepidoptera, Lycaenidae) from the Caucasus Íîâûå ñâåäåíèÿ î ìàëîèçó÷åííîì è ðåäêîì âèäå Athamanthia japhetica (Nekrutenko et Effendi, 1983) (Lepidoptera, Lycaenidae) íà Êàâêàçå D.V. Morgun Ä.Â. Ìîðãóí Moscow Centre of Environmental Education, Regional Research and Tourism, Odesskaya Str. 12A, Moscow 117303 Russia. E-mail: [email protected]. Московский центр экологии, краеведения и туризма, ул. Одесская 12А, Москва 117303 Россия. Key words: Athamanthia japhetica, Lepidoptera, Lycaenidae, distribution, Georgia, fauna. Ключевые слова: Athamanthia japhetica, Lepidoptera, Lycaenidae, распространение, Грузия, фауна. Abstract. New data on the distribution and ecology of the species distribution was treated as Apsheron peninsu- rare Lycaenid species Athamanthia japhetica (Nekrutenko et la in Caspian area of Azerbaijan [Tuzov et al., 2000]. In Effendi, 1939 (Lepidoptera, Lycaenidae) in the Central Cau- 2012 and 2013, V.V. Tikhonov and V.A. Lukhtanov found casus are presented. A comparison of the new material with the population of A. japhetica near the type locality in the specimens of the former known populations is described. the Tugchai valley, 30 km SW Kilyazy village, and near Резюме. Представлены новые сведения о распростра- Shirvan station. нении и экологии редкого вида голубянок (Athamanthia New population of A. japhetica in Georgia. Until japhetica (Nekrutenko et Effendi, 1983) (Lepidoptera, now, A. japhetica was treated as a species of Caspian Lycaenidae) в центральной части Кавказа. Описано срав- area of Eastern Azerbaijan, known only by two recently нение особей с особями ранее известных популяций вида. -

An Analysis of Heracles As a Tragic Hero in the Trachiniae and the Heracles
The Suffering Heracles: An Analysis of Heracles as a Tragic Hero in The Trachiniae and the Heracles by Daniel Rom Thesis presented for the Master’s Degree in Ancient Cultures in the Faculty of Arts and Social Sciences, at Stellenbosch University Supervisor: Prof. Annemaré Kotzé March 2016 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. March 2016 Copyright © 2016 Stellenbosch University All rights reserved Stellenbosch University https://scholar.sun.ac.za Abstract This thesis is an examination of the portrayals of the Ancient Greek mythological hero Heracles in two fifth century BCE tragic plays: The Trachiniae by Sophocles, and the Heracles by Euripides. Based on existing research that was examined, this thesis echoes the claim made by several sources that there is a conceptual link between both these plays in terms of how they treat Heracles as a character on stage. Fundamentally, this claim is that these two plays portray Heracles as a suffering, tragic figure in a way that other theatre portrayals of him up until the fifth century BCE had failed to do in such a notable manner. This thesis links this claim with a another point raised in modern scholarship: specifically, that Heracles‟ character and development as a mythical hero in the Ancient Greek world had given him a distinct position as a demi-god, and this in turn affected how he was approached as a character on stage. -

Roman Mythology Table of Contents
Roman Mythology Table of Contents • Historical Overview • Major Gods and Goddesses of ancient Rome • Gods of Mt Olympus • Family Tree • Conclusion Historical Overview • The early Romans were farmers • They did not understand science, instead they believed in forces or spirits • These Gods did not have human forms or minds • Gradually the Romans were influenced by the Greeks and adopted many of their Gods and gave them Roman names Early Roman Gods and Goddesses • Some Early Roman Gods or “Numen” – Bellona Goddess of War – Cardea similar to Artemis, had power over doorways – Cupid God of love like the Greek Eros – Dis God of the underworld like the Greek Hades – Faunus God of fields and shepards like the Greek Pan – Glaucus God of the sea – Lares God of the household- represented dead ancestors who protected the family – Liber God of fertility, identified with Baccus, the god of wine – Saturn God of agriculture and the Roman like the Greek Cronus – Vesta Goddess of home and hearth similar to the Greek Hestia Jupiter • King of the Gods/Ruler of the Skies • Greek name is Zeus • Married to Juno • Symbols: Eagle, Shield, Thunderbolt and Oak Tree Neptune • God of the Sea • Second most powerful God on Mt.Olympus • Greek name is Poseidon • Symbols: Trident, Horse and Bull Pluto • God the under world and wealth • Greek name is Hades • Symbols: Helmet, Metals, Jewels Juno • Queen of the Gods • Goddess of Heaven, Marriage and Childbirth • Greek name is Hera • Married to Jupiter • Symbols: Peacock and Cow Vesta • Goddess of the Hearth. Protector of -

Foraging Behaviour and Nectar Use in Adult Large Copper Butterflies, Lycaena Dispar (Lepidoptera: Lycaenidae)
© Entomologica Fennica. 27 May 2010 Foraging behaviour and nectar use in adult Large Copper Butterflies, Lycaena dispar (Lepidoptera: Lycaenidae) Marek B¹kowski, Agnieszka Filipiak & Zdenek Fric B¹kowski, M., Filipiak, A. & Fric, Z. 2010: Foraging behaviour and nectar use in adult Large Copper Butterflies, Lycaena dispar (Lepidoptera: Lycaenidae). Entomol. Fennica 21: 4957. The foraging behaviour of the endangered butterfly Lycaena dispar Haw. was examined in a wet meadow in Poznañ (western Poland) in the summer of 2003. Observations showed that the males spent more time resting (11.3% compared to 5.9%) and less time nectaring (24.8% compared 35%) compared to females. The mean time of one visit on a flower was almost three times shorter in males than in females. In total, adults visited flowers of nine nectar plant species, the most fre- quentones were Inula britannica, Lychnis flos-cuculi and Cirsium arvense, which were some of themostabundantplantspecies there.We observed differ- ences of nectar plant use between sexes and generations of the butterfly, but did notconfirm preference for theplantcolour. M. B¹kowski, Department of Systematic Zoology, Institute of Environmental Biology, A. Mickiewicz University, Umultowska 89, Poznañ 61-614, Poland; E- mail: [email protected] A. Filipiak, ul. Dêbowa 49/12 Poznañ, Poland Z. Fric, Biology Centre of the Czech Academy of Sciences, Institute of Entomolo- gy, Branisovska 31, CZ-37005 Ceske Budejovice, Czech Republic Received 27 May 2009, accepted 3 November 2009 1. Introduction 1978, Shreeve 1992). In addition, the differences in colour vision and recognition were repeatedly Butterflies are often considered to be opportunis- recorded even in butterflies of the studied genus tic foragers that visit a wide variety of available Lycaena (Bernard & Remington 1991). -

The Piercing of Glaucus Iliad 16.508-16.547 Jason Steranko
the piercing of glaucus Iliad 16.508-16.547 Jason Steranko, ‘17 terrible grief seized glaucus when he heard the dying voice, his heart was struck he could not save Sarpedon. he grasped his own throbbing arm and pressed hard, his wound wearying, the wound that teucer had dealt when from the high wall he let his arrow fly, defending his comrades from the lycian attacker. glaucus prayed to far-shooting apollo: ‘hear me, lord, wherever you may be, back in rich lycia, or here at troy: you are a god who hears from all directions the cries of grieving men: grief has come to me. a grievous wound overwhelms my arm, too deep to dry. it weighs down my shoulder and deadens my hand, too weak to take up my spear, too weak to avenge Sarpedon. he, our best man, the son of zeus, lies dead on the ground, abandoned by his father. lord apollo, heal this mighty wound of mine, lull my pains and grant me might that i may fight and marshal the scattered lycians and that i may guard whatoncewas Sarpedon.’ he spoke his prayer and apollo listened. he soothed the throbbing arm and dried black the dark and deep wound with his gleaming hand. the aching heart of glaucus surged with strength. the mortal knew it was the touch of the god, the quick touch of apollo, who heard his prayer. glaucus arose and inspired the leaders of lycia, gathered from across the battlefield, to surround their prostrate king, fallen Sarpedon. with purpose he marched to the trojan troop, to polydamas, son of panthous, and shining agenor. -

Federal Register/Vol. 76, No. 72/Thursday, April 14, 2011/Proposed Rules
20918 Federal Register / Vol. 76, No. 72 / Thursday, April 14, 2011 / Proposed Rules not provide information indicating how require empirical proof of a threat. The DEPARTMENT OF THE INTERIOR climate change might potentially impact combination of exposure and some the prairie chub. The prairie chub has corroborating evidence of how the Fish and Wildlife Service persisted for millennia with periods of species is likely impacted could suffice. extreme weather events, such as The mere identification of factors that 50 CFR Part 17 droughts and floods. If climate change could impact a species negatively may [Docket No. FWS–R8–ES–2010–0031; MO causes more extreme weather events, not be sufficient to compel a finding 92210–0–0008–B2] there is no information to indicate that that listing may be warranted. The such events will have a negative impact information must contain evidence Endangered and Threatened Wildlife on the prairie chub. At this time, we sufficient to suggest that these factors and Plants; 12-Month Finding on a lack sufficient certainty to know may be operative threats that act on the Petition To List Hermes Copper specifically how climate change will species to the point that the species may Butterfly as Endangered or Threatened affect the species. We are not aware of meet the definition of threatened or any data at an appropriate scale to endangered under the Act. AGENCY: Fish and Wildlife Service, evaluate habitat or population trends for Interior. Because we have found that the the prairie chub within its range, make ACTION: Notice of 12-month petition petition presents substantial predictions about future trends, or finding. -

Lycaena Hermes)
RARE BUTTERFLY MANAGEMENT STUDIES ON CONSERVED LANDS IN SAN DIEGO COUNTY: HERMES COPPER (LYCAENA HERMES) Translocation Final Report Prepared for: San Diego Association of Governments Contract: #5004388 Contract Manager: Keith Greer Prepared by: Dr. Daniel Marschalek and Dr. Douglas Deutschman, PI Department of Biology, San Diego State University 5 August 2016 Executive Summary The Hermes copper (Lycaena hermes) is a rare butterfly endemic to San Diego County and northern Baja California. This species is threatened by recent urbanization and wildfires throughout its range in the United States. In April of 2011 the United States Fish and Wildlife Service (USFWS) issued a 12-month finding which concluded that listing the Hermes copper butterfly as threatened or endangered was warranted, and is currently on the USFWS list of candidate species (USFWS 2011). Our research has documented several extirpations due to the 2003 and 2007 wildfires, but few recolonizations despite what appears to be suitable habitat. Although a few small populations exist within and north of the city of San Diego, the majority of Hermes copper individuals are found to the east and southeast of the city between the footprints of 2003 and 2007 fires. Due to the extremely restricted distribution, the species is highly vulnerable since one large fire could push the species to the brink of extinction. Recolonization into post-wildfire habitats is essential for the long-term persistence of Hermes copper; however, it appears that habitat fragmentation is limiting dispersal and preventing recolonizations from occurring. For these reasons, we initiated a project to evaluate translocation as a management tool for establishing self-sustaining Hermes copper populations.