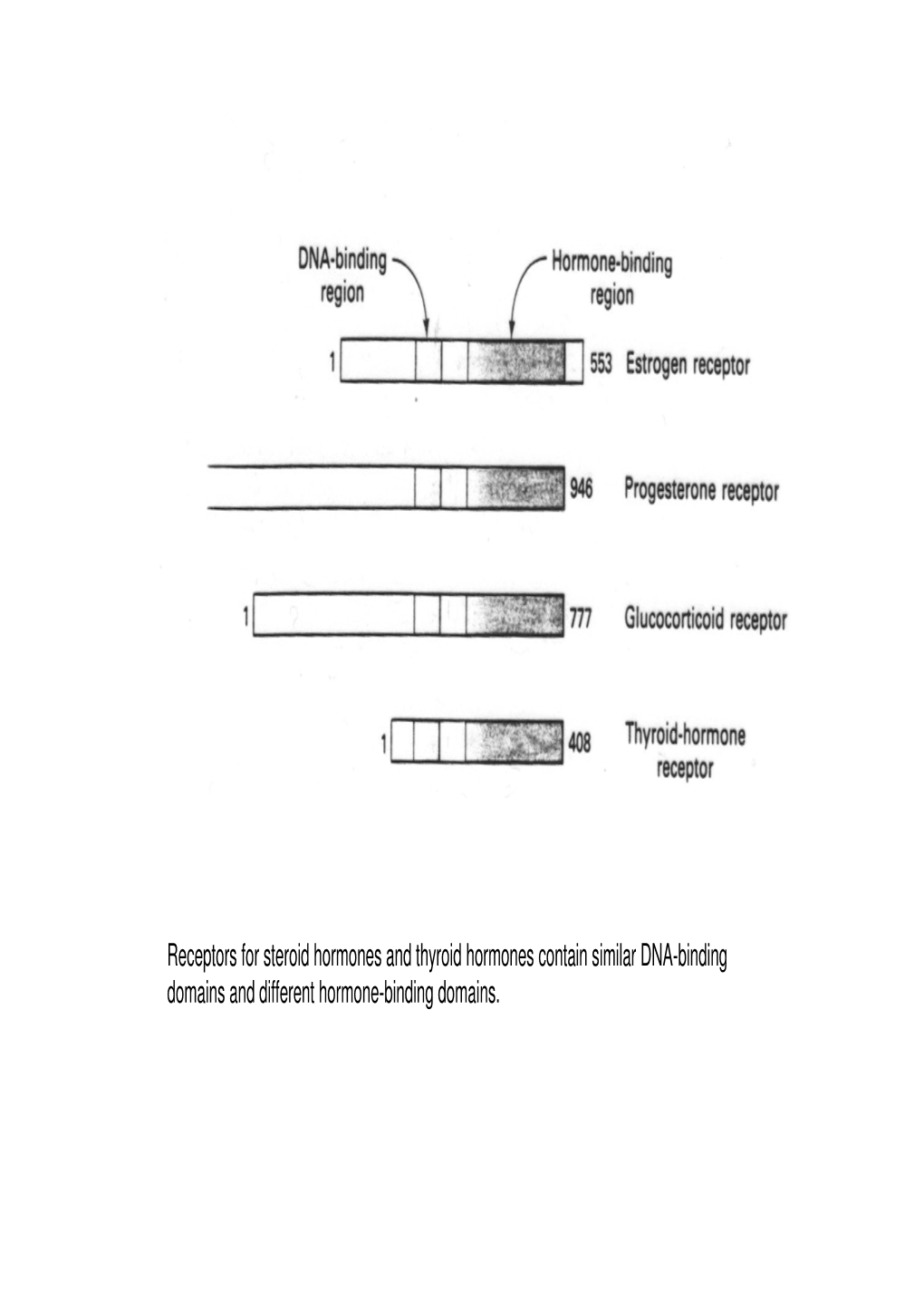
Receptors for Steroid Hormones and Thyroid Hormones Contain Similar DNA-Binding Domains and Different Hormone-Binding Domains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Convergence of Multiple Mechanisms of Steroid Hormone Action
Review 569 Convergence of Multiple Mechanisms of Steroid Hormone Action Authors S. K. Mani 1 * , P. G. Mermelstein 2 * , M. J. Tetel 3 * , G. Anesetti 4 * Affi liations 1 Department of Molecular & Cellular Biology and Neuroscience, Baylor College of Medicine, Houston, TX, USA 2 Department of Neuroscience, University of Minnesota, Minneapolis, MN, USA 3 Neuroscience Program, Wellesley College, Wellesley, MA, USA 4 Departamento de Hostologia y Embriologia, Facultad de Medicine, Universidad de la Republica, Montevideo, Uruguay Key words Abstract receptors can also be activated in a “ligand-inde- ● ▶ estrogen ▼ pendent” manner by other factors including neu- ● ▶ progesterone Steroid hormones modulate a wide array of rotransmitters. Recent studies indicate that rapid, ▶ ● signaling physiological processes including development, nonclassical steroid eff ects involve extranuclear ● ▶ cross-talk metabolism, and reproduction in various species. steroid receptors located at the membrane, which ● ▶ ovary ● ▶ brain It is generally believed that these biological eff ects interact with cytoplasmic kinase signaling mol- are predominantly mediated by their binding to ecules and G-proteins. The current review deals specifi c intracellular receptors resulting in con- with various mechanisms that function together formational change, dimerization, and recruit- in an integrated manner to promote hormone- ment of coregulators for transcription-dependent dependent actions on the central and sympathetic genomic actions (classical mechanism). In addi- nervous systems. tion, to their cognate ligands, intracellular steroid Abbreviations gene expression and function. Interestingly, not ▼ all the “classical” receptors are intranuclear and CBP CREB binding protein can be associated at the membrane. As described CRE CREB response element in this review, extranuclear ERs and PRs at the DAR Dopamine receptor (DAR) membrane or in the cytoplasm can interact with ER Estrogen receptor G proteins and signaling kinases, and other G received 13 . -

Steroid Hormones
Chemical Signaling Hormonal Signal Transduction Pathways Types of Chemical Signaling Chemical signaling between cells is one of the most important ways that activities of tissues and organs are coordinated. The nervous system is the other major coordinating system in animals, but even here chemical signaling is used between adjacent neurons. Three major types: Sites of Hormone Release More commonly, especially in the vertebrates, the sources of hormones are specialized glands called endocrine glands. Even here the source of the hormone is often modified nerve cells or neurosecretory cells. Some organs that have other functions also release hormones - for example, many hormones are released by different organs of the digestive system. Types of Responses Controlled by Hormones Hormones are not only used for long-distance signaling around the body, but they also provide for more prolonged regulatory responses. Prolonged responses can persist for minutes, hours, or even many days. Examples include maintenance of blood osmolarity, blood glucose, metabolic rate of specific tissues, control of reproductive cycles. The nervous system is used for more rapid responses of shorter duration. Example of Regulation of Blood Glucose Types of Hormones 1) Amines: Catecholamines, epinephrine and norepinephrine, derived from amino acids 2) Prostaglandins: Cyclic unsaturated fatty acids Types of Hormones 3) Steroid hormones: Include testosterone and estrogen, cyclic hydrocarbons derived from cholesterol. 4) Peptides and proteins: Include insulin Three Steps in Cell Signaling Target organ specificity is the result of specific receptor molecules for the hormone, either on the plasma membrane surface, or in some cases in the cytoplasm, of cells in the target organ. -

The Orphan Receptor Rev-Erbaot Activates Transcription Via a Novel Response Element HEATHER P
MOLECULAR AND CELLULAR BIOLOGY, May 1993, p. 3113-3121 Vol. 13, No. 5 0270-7306/93/053113-09$02.00/0 Copyright © 1993, American Society for Microbiology The Orphan Receptor Rev-ErbAot Activates Transcription via a Novel Response Element HEATHER P. HARDING AND MITCHELL A. LAZAR* Endocrinology Division, Department ofMedicine, and Department of Genetics, University ofPennsylvania School ofMedicine, Philadelphia, Pennsylvania 19104 Received 18 December 1992/Returned for modification 1 February 1993/Accepted 11 February 1993 Rev-ErbAca (Rev-Erb) is a nuclear hormone receptor-related protein encoded on the opposite strand of the at-thyroid hormone receptor (TR) gene. This unusual genomic arrangement may have a regulatory role, but the conservation of human and rodent Rev-Erb amino acid sequences suggests that the protein itself has an important function, potentially as a sequence-specific transcriptional regulator. However, despite its relation- ship to the TR, Rev-Erb bound poorly to TR binding sites. To determine its DNA-binding specificity in an unbiased manner, Rev-Erb was synthesized in Escherichia coli, purified, and used to select specific binding sites from libraries of random double-stranded DNA sequences. We found that Rev-Erb binds to a unique site consisting of a specific 5-bp A/T-rich sequence adjacent to a TR half-site. Rev-Erb contacts this entire asymmetric 11-bp sequence, which is the longest nonrepetitive element specifically recognized by a member of the thyroid/steroid hormone receptor superfamily, and mutations in either the A/T-rich or TR half-site regions abolished specific binding. The binding specificity of wild-type Rev-Erb was nearly identical to that of C- and N-terminally truncated forms. -

Chapter 9 Cell Signaling Events
Chapter 9 Cell Signaling Events © 2020 Elsevier Inc. All rights reserved. Figure 9–1. Fibroblast growth factor (FGF) signal transduction pathways. Activated FGF receptors (FGFRs; red rectangles) stimulate the phospholipase Cγ (PLCγ) pathway (blue highlight), the phosphatidylinositol 3-kinase (PI3K)-AKT/protein kinase B (PKB) pathway (yellow highlight), and the FRS2-RAS-mitogen-activated protein kinase (MAPK) pathway (green highlight). The activated MAPKs (extracellular signal-regulated kinases (ERKs), p38, or c-Jun N-terminal kinases (JNKs)) are translocated to the nucleus where they phosphorylate (P) transcription factors, thereby regulating target genes. (Modified from Dailey L, et al. Cytokine Growth Factor Rev 2005;16:233, by permission.) © 2020 Elsevier Inc. All rights reserved. 2 Figure 9–2. Activation and feedback regulation of the MAPK pathway. The classical MAPK pathway is activated in human tumors by upstream receptor tyrosine kinases (RTKs) or by mutations in RAS, BRAF, and MEK1. RTKs activate RAS by recruiting adaptor proteins (e.g., GRB-2) and exchange factors (e.g., Sos). RAS activation promotes the formation of RAF dimers, which activate MEK-ERK cascade through phosphorylation. ERK pathway activity is regulated by negative feedback at multiple levels, including the transcriptional activation of DUSP proteins that negatively regulate the pathway. ERK also phosphorylates and thus regulates CRAF and MEK activity directly. ERK, or its immediate substrate RSK, also phosphorylates Sos at several residues, inhibiting its activity and thus negatively regulating RAS activity. (From Liu, et al., Acta Pharm Sin B 2018;8(4):552–562.) © 2020 Elsevier Inc. All rights reserved. 3 Figure 9–3. PI3 kinase-AKT pathway mutations in cancer. -

Identification of a Novel Sex Steroid Binding Protein (Receptors/Sterophilin/Chick Oviduct) ROBERT N
Proc. NatL Acad. Sci. USA Vol. 79, pp. 1742-1746, March 1982 Biochemistry Identification of a novel sex steroid binding protein (receptors/sterophilin/chick oviduct) ROBERT N. TAYLOR* AND RoY G. SMITHt Division of Urology (Surgery), and Department of Biochemistry and Molecular Biology, The University ofTexas Medical School at Houston, Houston, Texas 77030 Communicated by Elwood V. Jensen, November 25, 1981 ABSTRACT We describe a cytoplasmic steroid binding pro- senting the species with the higher affinity. This report de- tein in the chick oviduct which has intriguing characteristics. This scribes the identification of a new estrogen binding protein, protein binds [3H]estradiol with high affinity (Kd = 30 x 10-9 M) designated Z. and limited capacity (300 fmol/mg ofcytosol protein). It sediments at 8 S in low-salt sucrose density gradients and at 4 S in high-salt MATERIALS AND METHODS gradients. Unlike the estrogen receptor, however, this protein also Animals. Two-day-old White Leghorn pullets were pur- binds progesterone, R5020, testosterone, and 5a-dihydrotestos- chased from Texas Animal Specialties (Humble, TX), provided terone with similar affinities and in a competitive manner. More- with food and water ad lib, and raised in a constant temperature over, it is not translocated to the nucleus by the in vivo adminis- environment with a 13/11-hr light/dark schedule. At the chick tration of these sex steroids. The protein is only present in age of1 wk, estrogen stimulation was initiated by subcutaneous estrogen-responsive tissue, and like the sex steroid receptors, its insertion of two silicone implants containing 25 mg of diethyl- synthesis appears to be regulated by estrogen. -

And Steroid Hormone Receptor Activity in Cancer
37 REVIEW Sirtuin 1 (SIRT1) and steroid hormone receptor activity in cancer R L Moore1, Y Dai1,2 and D V Faller1,2,3,4,5,6 1Cancer Center, Departments of 2Medicine, 3Biochemistry, 4Pediatrics, 5Microbiology and 6Pathology and Laboratory Medicine, Boston University School of Medicine, 72 East Concord Street, Room K-701, Boston, Massachusetts 02118-2307, USA (Correspondence should be addressed to D V Faller at Boston University School of Medicine; Email: [email protected]) Abstract Sirtuins, which are class III NAD-dependent histone signaling through a variety of molecular mechanisms, deacetylases that regulate a number of physiological processes, including acting as co-regulatory transcription factors, play important roles in the regulation of metabolism, aging, deacetylating histones in the promoters of genes with nuclear oncogenesis, and cancer progression. Recently, a role for receptor-binding sites, directly deacetylating steroid hormone the sirtuins in the regulation of steroid hormone receptor nuclear receptors, and regulating pathways that modify steroid signaling is emerging. In this mini-review, we will summarize hormone receptors through phosphorylation. Furthermore, current research into the regulation of estrogen, androgen, disruption of sirtuin activity may be an important step in the progesterone, mineralocorticoid, and glucocorticoid signaling development of steroid hormone-refractory cancers. by sirtuins in cancer. Sirtuins can regulate steroid hormone Journal of Endocrinology (2012) 213, 37–48 Introduction cancer, ovarian -

Topic 1.1 Nuclear Receptor Superfamily: Principles of Signaling*
Pure Appl. Chem., Vol. 75, Nos. 11–12, pp. 1619–1664, 2003. © 2003 IUPAC Topic 1.1 Nuclear receptor superfamily: Principles of signaling* Pierre Germain1, Lucia Altucci2, William Bourguet3, Cecile Rochette-Egly1, and Hinrich Gronemeyer1,‡ 1IGBMC - B.P. 10142, F-67404 Illkirch Cedex, C.U. de Strasbourg, France; 2Dipartimento di Patologia Generale, Seconda Università di Napoli, Vico Luigi De Crecchio 7, I-80138 Napoli, Italia; 3CBS, CNRS U5048-INSERM U554, 15 av. C. Flahault, F-34039 Montpellier, France Abstract: Nuclear receptors (NRs) comprise a family of 49 members that share a common structural organization and act as ligand-inducible transcription factors with major (patho)physiological impact. For some NRs (“orphan receptors”), cognate ligands have not yet been identified or may not exist. The principles of DNA recognition and ligand binding are well understood from both biochemical and crystal structure analyses. The 3D structures of several DNA-binding domains (DBDs), in complexes with a variety of cognate response elements, and multiple ligand-binding domains (LBDs), in the absence (apoLBD) and pres- ence (holoLBD) of agonist, have been established and reveal canonical structural organiza- tion. Agonist binding induces a structural transition in the LBD whose most striking feature is the relocation of helix H12, which is required for establishing a coactivator complex, through interaction with members of the p160 family (SRC1, TIF2, AIB1) and/or the TRAP/DRIP complex. The p160-dependent coactivator complex is a multiprotein complex that comprises histone acetyltransferases (HATs), such as CBP, methyltransferases, such as CARM1, and other enzymes (SUMO ligase, etc.). The agonist-dependent recruitment of the HAT complex results in chromatin modification in the environment of the target gene pro- moters, which is requisite to, or may in some cases be sufficient for, transcription activation. -

The Prognostic Impact of Retinoid X Receptor and Thyroid Hormone Receptor Alpha in Unifocal Vs
International Journal of Molecular Sciences Article The Prognostic Impact of Retinoid X Receptor and Thyroid Hormone Receptor alpha in Unifocal vs. Multifocal/Multicentric Breast Cancer Alaleh Zati Zehni 1, Falk Batz 1, Aurelia Vattai 1, Till Kaltofen 1 , Svenja Schrader 1, Sven-Niclas Jacob 2 , Jan-Niclas Mumm 3, Helene Hildegard Heidegger 1, Nina Ditsch 1,4, Sven Mahner 1, Udo Jeschke 1,4,* and Theresa Vilsmaier 1 1 Department of Obstetrics and Gynecology, University Hospital Munich, LMU, 80337 Munich, Germany; [email protected] (A.Z.Z.); [email protected] (F.B.); [email protected] (A.V.); [email protected] (T.K.); [email protected] (S.S.); [email protected] (H.H.H.); [email protected] (N.D.); [email protected] (S.M.); [email protected] (T.V.) 2 Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, LMU, Marchioninistraße 15, 81377 Munich, Germany; [email protected] 3 Department of Urology, LMU, Marchioninistraße 15, 81377 Munich, Germany; [email protected] 4 Department of Obstetrics and Gynecology, University Hospital, 86156 Augsburg, Germany * Correspondence: [email protected]; Tel.: +49-8214-0016-5505 Abstract: The aim of this study was to assess the prognostic value of the steroid hormone receptor expression, counting the retinoid X receptor (RXR) and thyroid hormone receptors (THRs), on the Citation: Zehni, A.Z.; Batz, F.; Vattai, two different breast cancer (BC) entities: multifocal/multicentric versus unifocal. -

Nuclear Receptors Product Listing | Edition 2
Nuclear Receptors Product Listing | Edition 2 Kudzu Pueraria lobata A source of Daidzein Nuclear Receptor Products by Group: • Steroid Hormone Receptor Group • Thyroid Hormone Receptor-like Group • Retinoid X Receptor-like Group • Steroidogenic Factor-like Group Tocris Product Listing Series Contents This listing contains over 150 products from Tocris, including a wide range of nuclear receptor agonists and antagonists available for each nuclear receptor group. Related products are also listed, alongside a selection of relevant scientific literature available from www.tocris.com. Introduction................................................................................... 3 Thyroid Hormone Receptor-like Group (continued) Pregnane X Receptor (PXR) ................................................. 9 Steroid Hormone Receptor Group ........................................... 5 Retinoic Acid Receptors (RAR)......................................... 10 Androgen Receptor (AR) ...................................................... 5 Rev-Erb Receptors ............................................................... 11 Estrogen Receptors (ER) ...................................................... 5 Thyroid Hormone Receptors (TR) .................................... 11 Estrogen-related Receptors (ERR) ..................................... 7 Vitamin D Receptor (VDR) ................................................. 11 ............................................. Glucocorticoid Receptor (GR) 7 Retinoid X Receptor-like Group ........................................... -

Nuclear Receptors and Development of Marine Invertebrates Angelica Miglioli 1,2, Laura Canesi 2, Isa D
G C A T T A C G G C A T genes Review NuclearReview Receptors and Development of Marine Invertebrates Nuclear Receptors and Development of Marine Invertebrates Angelica Miglioli 1,2, Laura Canesi 2, Isa D. L. Gomes 1, Michael Schubert 1 and Rémi Dumollard 1,* Angelica Miglioli 1,2 , Laura Canesi 2 , Isa D. L. Gomes 1, Michael Schubert 1 and Rémi Dumollard 1,* 1 Laboratoire de Biologie du Développement de Villefranche-sur-Mer (LBDV), Institut de la Mer de Ville- franche, Sorbonne Université, CNRS, 181 Chemin du Lazaret, 06230 Villefranche-sur-Mer, France; miglio- 1 Laboratoire de Biologie du Développement de Villefranche-sur-Mer (LBDV), Institut de la Mer de Villefranche, [email protected] (A.M.); [email protected] (I.D.L.G.); [email protected] (M.S.) Sorbonne Université, CNRS, 181 Chemin du Lazaret, 06230 Villefranche-sur-Mer, France; 2 Dipartimento di Scienze della Terra, dell’Ambiente e della Vita (DISTAV), Università degli Studi di Genova, [email protected] (A.M.); [email protected] (I.D.L.G.); [email protected] (M.S.) Corso Europa 26, 16132 Genova, Italy; [email protected] 2 Dipartimento di Scienze della Terra, dell’Ambiente e della Vita (DISTAV), Università degli Studi di Genova, * Correspondence: [email protected] Corso Europa 26, 16132 Genova, Italy; [email protected] * Correspondence: [email protected] Abstract: Nuclear Receptors (NRs) are a superfamily of transcription factors specific to metazoans that have the unique ability to directly translate the message of a signaling molecule into a tran- Abstract: Nuclear Receptors (NRs) are a superfamily of transcription factors specific to metazoans scriptional response. -

Molecular Dynamics of Estrogen Receptors
DOI: 10.14744/ejmo.2018.76476 EJMO 2018;2(4):189–197 Review Molecular Dynamics of Estrogen Receptors Zehra Okat Uskudar Zeynep Kamil Vocational and Technical Anatolian High School, Istanbul, Turkey Abstract Nuclear receptor (NR) superfamily is located in the transcriptional regulator class. Owing to their important role in controlling many physiological and pathological events, it has become the most important therapeutic targets in clinical trials. Although it is used successfully in many cases, allowing receptor-modulating drugs, owing to targeted therapy resistance, the mechanisms of NRs that work for generating new drugs are still up to date. Most success- ful target therapy for controlling the activity of the receptor was conducted based on the NR signaling pathway. In this review, estrogen receptor (ER) subtypes, ER domain structure and general features, ER molecular signaling mechanisms, ER degradation occurring with the ubiquitin–proteasome pathway, ER degradation triggered by basal and ligand, effect of ER concentration in response to estrogen, and ER alpha molecular background of the action of agonists and antagonists are explained in detail. The comprehensive information in this article is intended to provide a clearer understanding of the receptor function in the control of key points. We believe that it would be useful for future therapeutic approaches. Keywords: Nuclear receptors, ER alpha, ER beta, ER degradation Cite This Article: Okat Z. Molecular dynamics of estrogen receptors. EJMO. 2018; 2(4): 189-197 strogens are cyclopentafenanthene compounds. Estro- Estrogen Receptors (ERs) gens are synthesized from cholesterol, like sex and ad- E Estrogens are passively diffuse to cytoplasmic and nuclear renal hormones. -

P53: Protection Against Tumor Growth Beyond Effects on Cell Cycle and Apoptosis Xuyi Wang1,2, Evan R
Published OnlineFirst November 16, 2015; DOI: 10.1158/0008-5472.CAN-15-0563 Cancer Review Research p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis Xuyi Wang1,2, Evan R. Simpson1,3, and Kristy A. Brown1,2 The tumor suppressor p53 has established functions in cancer. lication by Wang and colleagues demonstrates for the first time Specifically, it has been shown to cause cell-cycle arrest and that p53 is a key negative regulator of aromatase and, hence, apoptosis in response to DNA damage. It is also one of the most estrogen production in the breast tumor microenvironment. It commonly mutated or silenced genes in cancer and for this reason goes further by demonstrating that an important regulator of has been extensively studied. Recently, the role of p53 has been aromatase, the obesity-associated and tumor-derived factor pros- shown to go beyond its effects on cell cycle and apoptosis, with taglandin E2, inhibits p53 in the breast adipose stroma. This effects on metabolism emerging as a key contributor to cancer review presents these findings in the context of established and growth in situations where p53 is lost. Beyond this, the role of p53 emerging roles of p53 and discusses possible implications for the in the tumor microenvironment is poorly understood. The pub- treatment of breast cancer. Cancer Res; 75(23); 5001–7. Ó2015 AACR. Background induces apoptosis. The mitochondrial pathway is mainly reg- ulated by p53 effector Bcl-2 proteins such as Bax (4) and PUMA Traditional tumor suppressor functions of p53 (5). In tumors with wild-type p53, p53 responses can be The tumor suppressor p53, encoded by the TP53 gene, is the inhibited by downregulating p53 activity or its effectors' activ- most commonly silenced or mutated gene in cancer, with 50% to þ ity.