Atrioventricular Septal Defects (AV Canal Defect, Endocardial Cushion Defects)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart and Circulatory System I
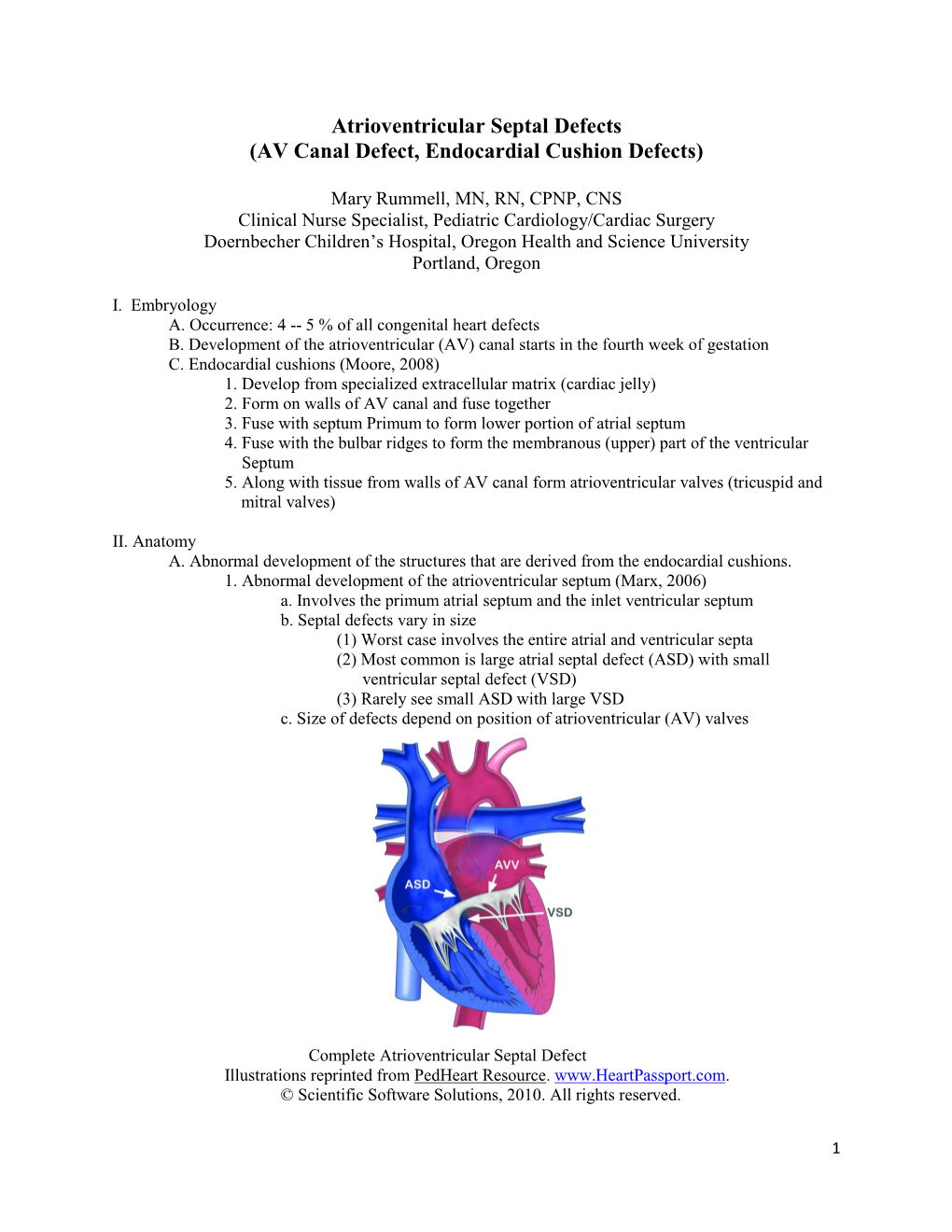
Heart and Circulatory System I Daphne T. Hsu, MD Professor of Clinical Pediatrics [email protected] Outline • Vasculogenesis • Embryonic Folding • Formation of the Primary Heart Tube • Looping • Atrial Septation • Primitive Ventricular Septum • Atrioventricular Canal/Endocardial Cushions • Conotruncal Septation • Ventricular septation • Congenital Heart Defects CARDIOVASCULAR SYSTEM: EARLY DEVELOPMENT: WEEK 3 EMBRYONIC FOLDING: WEEK 4 Formation of Heart Tube (17-22 days) Heart Development: 26 days PRIMITIVE HEART TUBE: WEEK 4 Cardiac Loop (8-16 somites) VENTRICULAR LOOPING END WEEK 4 NORMAL : Loop to the RIGHT: Levocardia! ABNORMAL: Loop to the LEFT: Dextrocardia! FROM TUBE TO FOUR CHAMBERS INTERNAL VIEW Formation of Primitive Ventricles Atrial Septation: 3 Septums Primum, Secundum, Intermedium Endocardial Cushion: 80 days Endocardial Cushions • Atrioventricular Canal: Divide between the atria and ventricles • Endocardial Cushions: Four tissue expansions found in periphery of AV canal – Atrial septation – Atrioventricular valve formation: Mitral and Tricuspid Valves – Ventricular septation Endocardial Cushions • Superior-Inferior cushions – Septum Intermedium – Inferior atrial septum – Posterior/superior ventricular septum • Right and Left Cushions – Ventricular myocardium – Mitral valve – Tricuspid valve Atrioventricular Valve Formation • Left and Right Endocardial Cushions FOUR CHAMBERS- ULTRASOUND VIEW @ 20 wks Congenital Heart Defect: Endocardial Cushion Defect Normal Endocardial Cushion Defect Ventricular Outflow Tracts and Great -

The Zebrafish Cardiac Endothelial Cell—Roles in Development And
Journal of Cardiovascular Development and Disease Review The Zebrafish Cardiac Endothelial Cell—Roles in Development and Regeneration Vanessa Lowe 1, Laura Wisniewski 2 and Caroline Pellet-Many 3,* 1 Heart Centre, Barts & The London School of Medicine, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; [email protected] 2 Centre for Tumour Microenvironment, Barts Cancer Institute, Queen Mary University London, Charterhouse Square, London EC1M 6BQ, UK; [email protected] 3 Department of Comparative Biomedical Sciences, Royal Veterinary College, 4 Royal College Street, London NW1 0TU, UK * Correspondence: [email protected] Abstract: In zebrafish, the spatiotemporal development of the vascular system is well described due to its stereotypical nature. However, the cellular and molecular mechanisms orchestrating post-embryonic vascular development, the maintenance of vascular homeostasis, or how coronary vessels integrate into the growing heart are less well studied. In the context of cardiac regeneration, the central cellular mechanism by which the heart regenerates a fully functional myocardium relies on the proliferation of pre-existing cardiomyocytes; the epicardium and the endocardium are also known to play key roles in the regenerative process. Remarkably, revascularisation of the injured tissue occurs within a few hours after cardiac damage, thus generating a vascular network acting as a scaffold for the regenerating myocardium. The activation of the endocardium leads to the secretion of cytokines, further supporting the proliferation of the cardiomyocytes. Although epicardium, Citation: Lowe, V.; Wisniewski, L.; endocardium, and myocardium interact with each other to orchestrate heart development and Pellet-Many, C. The Zebrafish Cardiac regeneration, in this review, we focus on recent advances in the understanding of the development of Endothelial Cell—Roles in the endocardium and the coronary vasculature in zebrafish as well as their pivotal roles in the heart Development and Regeneration. -

Atrioventricular Canal Defects: Morphologic Features and Surgical Techniques
ISSN: 2574-1241 Volume 5- Issue 4: 2018 DOI: 10.26717/BJSTR.2018.10.001930 Koray Ak. Biomed J Sci & Tech Res Review Article Open Access Atrioventricular Canal Defects: Morphologic Features and Surgical Techniques Koray Ak* Department of Cardiovascular Surgery, Marmara University School of Medicive, Turkey Received: Published: *Corresponding: September author: 29, 2018; : October 23, 2018 Koray Ak, Kalp ve Damar Cerrahisi AD, Sağlık Bakanlığı Marmara Universitesi, Pendik Egitim ve Arastirma Hastanesi, 4. Kat, Ustkaynarca Pendik, Istanbul, Turkey Abbreviations: International Pediatric And Congenital Cardiac Coding (IPCCC); Atrial Septal Defect (ASD); Cardiopulmonary Bypass (CPB) Introduction with partial AV canals. In AV canal defects, aortic valve loses its After the first successful repair of an AV canal defect by C. wedged position between the left and the right AV valves. Walton Lillehei in 1955, there have been tremendous improvements in both surgical techniques and perioperative care of patients. From surgical point of view, one of the most important Nowadays early mortality after surgical repair has dropped to less morphological features of AV canal defects is the location of 3 to 4 % in many parts of the world. On the other hand, in terms the conduction system. Here, atrioventricular node is displaced of complexity, the repair of an AV canal defect could be quite inferiorly and posteriorly toward the coronary sinus due to the challenging for surgeons in some cases. When we look at Aristotle presence of an ostium primum ASD (Figure 1). With this regard, a scoring system which grades congenital cardiac procedures with special attention should be paid during surgical repair in order to regard to their complexity (simple: 1.5 to very complex: 15), prevent the development of complete AV block. -

The Transcription Factor Sox7 Modulates Endocardiac Cushion
Hong et al. Cell Death and Disease (2021) 12:393 https://doi.org/10.1038/s41419-021-03658-z Cell Death & Disease ARTICLE Open Access The transcription factor Sox7 modulates endocardiac cushion formation contributed to atrioventricular septal defect through Wnt4/ Bmp2 signaling Nanchao Hong1,2, Erge Zhang1, Huilin Xie1,2,LihuiJin1,QiZhang3, Yanan Lu1,AlexF.Chen3,YongguoYu4, Bin Zhou 5,SunChen1,YuYu 1,3 and Kun Sun1 Abstract Cardiac septum malformations account for the largest proportion in congenital heart defects. The transcription factor Sox7 has critical functions in the vascular development and angiogenesis. It is unclear whether Sox7 also contributes to cardiac septation development. We identified a de novo 8p23.1 deletion with Sox7 haploinsufficiency in an atrioventricular septal defect (AVSD) patient using whole exome sequencing in 100 AVSD patients. Then, multiple Sox7 conditional loss-of-function mice models were generated to explore the role of Sox7 in atrioventricular cushion development. Sox7 deficiency mice embryos exhibited partial AVSD and impaired endothelial to mesenchymal transition (EndMT). Transcriptome analysis revealed BMP signaling pathway was significantly downregulated in Sox7 deficiency atrioventricular cushions. Mechanistically, Sox7 deficiency reduced the expressions of Bmp2 in atrioventricular canal myocardium and Wnt4 in endocardium, and Sox7 binds to Wnt4 and Bmp2 directly. Furthermore, 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; WNT4 or BMP2 protein could partially rescue the impaired EndMT process caused by Sox7 deficiency, and inhibition of BMP2 by Noggin could attenuate the effect of WNT4 protein. In summary, our findings identify Sox7 as a novel AVSD pathogenic candidate gene, and it can regulate the EndMT involved in atrioventricular cushion morphogenesis through Wnt4–Bmp2 signaling. -

Anatomical-Embryological Correlates in Atrioventricular Septal Defect
Br Heart J: first published as 10.1136/hrt.47.5.419 on 1 May 1982. Downloaded from Br Heart J 1982; 47: 419-29 Anatomical-embryological correlates in atrioventricular septal defect SALLY P ALLWORK* From the Department ofSurgery, Division of Cardiovascular Disease, Royal Postgraduate Medical School, Hammersmith Hospital, London SUMMARY Recent embryological studies have supported the consideration that the ventricular sep- tum is multifocal in origin. These data have also provided excellent correlation of the morphology of malformed hearts with their embryology. In particular, atrioventricular septal defect correlates accurately with these observations on ventricular septation. Many of the names given to atrioven- tricular septal defect (for example ostium primum, persistent atrioventricular canal, endocardial cushion defect) indicate attempts at correlating the anatomy with embryology. None of these has been very convincing. In the light of this uncertainty, this review considers briefly the anatomy of the malformation and its ontogeny, and presents a hypothesis of the development of atrioventricular septal defect. Although there is almost always a communication above the atrioventricular valves, the malforma- tion lies in the ventricular, not the atrial septum. Hearts with inlet septal defect without interatrial communication represent one end of the spectrum of anomalies, and those with common atrioven- tricular orifice, in which Fallot's tetralogy or single outlet heart may be associated, mark the other end. The outflow tract malformations are not randomly associated, but are points in a huge range of cardiac malformations. http://heart.bmj.com/ Atrioventricular septal defect is a cardiac malforma- leaflets themselves are often poorly developed and tion characterised externally by an abnormally short may have a verrucous appearance. -

Atrial Septal Defect
ATRIAL SEPTAL DEFECT BY D. EVAN BEDFORD, CORNELIO PAPP, AND JOHN PARKINSON Received October 21, 1940 Patent foramen ovale and atrial (or auricular) septal defect (A.S.D.), though both characterized by an aperture in the atrial septum, are embryologically and pathologically different conditions. Slit patency of the foramen ovale (to probe or even to pencil) is a common- place in a normal atrial septum. As it should close during the first year of life, the patent foramen ovale is scarcely to be regarded as a congenital cardiac lesion, but rather as an anatomical variation of a pre-existent condition (Costa, 1931). It is present in 20-30 per cent of all necropsies (Thompson & Evans, 1930; McGinn & White, 1936; O'Farrell, 1938), and it is clinically silent. In excep- tional circumstances an increase in the right atrial pressure during hypertensive cardiac failure (Marchal, Ortholan, & Breton, 1939) or in mitral stenosis (Lutem- bacher, 1916, 1936) can open up the slit foramen (" widely patent ") and determine or accentuate a terminal cyanosis. Pulmonary infarction may similarly enlarge the slit foramen and so facilitate the passage of a paradoxical embolus (Barnard, 1930; Thompson & Evans, 1930; Jones, 1936; Lofgren, 1937; Hirschboeck, 1935; Koritschoner, 1936). Terminal cyanosis and para- doxical embolism are the only two events referable to this common condition- a slit or widely patent foramen ovale-and they are rare. In contrast, an atrial septal defect is a malformation that is really con- genital. The development of the atrial septum by the union of three incomplete septa, namely, the septum primum (or superius), the septum secundum, and the septum intermedium, leaves pervious first the foramen ovale primum and later the foramen ovale secundum. -

Placental Pathology and Neuroimaging Correlates in Neonates with Congenital Heart Disease
www.nature.com/scientificreports OPEN Placental Pathology and Neuroimaging Correlates in Neonates with Congenital Heart Received: 15 November 2018 Accepted: 19 February 2019 Disease Published: xx xx xxxx Sarah D. Schlatterer1, Jonathan Murnick2, Marni Jacobs3, Linda White2, Mary T. Donofrio4,5 & Catherine Limperopoulos2,5 Congenital heart disease (CHD) is an independent risk factor for brain injury, including stroke, and poor neurodevelopmental outcomes, and placental abnormalities may represent an additional risk factor for brain injury in neonates. The incidence and scope of placental pathology and relationship to fetal brain abnormalities in pregnancies complicated by fetal CHD has not been explored to our knowledge. In order to determine the prevalence of placental pathology fndings and whether placental fndings are associated with postnatal brain injury in pregnancies complicated by fetal CHD, we reviewed placental pathology reports for 51 pregnancies complicated by CHD and scored available postnatal, pre-operative brain MRI for brain pathology. Overall, 57% of CHD infants had abnormal placental pathology. Pregnancies complicated by CHD with aortic obstruction (AO) were signifcantly more likely than those with no obstruction to have abnormal placental pathology (79% vs. 44%). There was a trend toward more severe brain lesions amongst patients with brain lesions and placental abnormality (55% moderate/severe) compared to those without placental abnormality (11% moderate/severe). These data suggest that placental abnormalities are common in CHD and may have a compounding efect on brain lesions in this high-risk population. Te incidence and scope of placental pathology and its relationship to fetal brain anomalies in pregnancies com- plicated by fetal congenital heart disease (CHD) is an area of study that is in its infancy. -

Identification and Morphogenesis of Vestibular Atrial Septal Defects
Journal of Cardiovascular Development and Disease Article Identification and Morphogenesis of Vestibular Atrial Septal Defects Rohit S. Loomba 1,* , Justin T. Tretter 2 , Timothy J. Mohun 3, Robert H. Anderson 4 , Scott Kramer 5 and Diane E. Spicer 5 1 Division of Cardiology, Advocate Children’s Hospital, Chicago, IL 60453, USA 2 Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; [email protected] 3 Francis Crick Institute, London NW1 1AT, UK; [email protected] 4 Cardiovascular Research Centre, Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne NE1 3BZ, UK; [email protected] 5 Division of Pediatric Cardiology, University of Florida, Gainesville, FL 32611, USA; [email protected] (S.K.); [email protected] (D.E.S.) * Correspondence: [email protected] Received: 2 May 2020; Accepted: 10 August 2020; Published: 10 September 2020 Abstract: Background: The vestibular atrial septal defect is an interatrial communication located in the antero-inferior portion of the atrial septum. Reflecting either inadequate muscularization of the vestibular spine and mesenchymal cap during development, or excessive apoptosis within the developing antero-inferior septal component, the vestibular defect represents an infrequently recognized true deficiency of the atrial septum. We reviewed necropsy specimens from three separate archives to establish the frequency of such vestibular defects and their associated cardiac findings, providing additional analysis from developing mouse hearts to illustrate their potential morphogenesis. Materials and methods: We analyzed the hearts in the Farouk S. Idriss Cardiac Registry at Ann and Robert H. Lurie Children’s Hospital in Chicago, IL, the Van Mierop Archive at the University of Florida in Gainesville, Florida, and the archive at Johns Hopkins All Children’s Heart Institute in St. -

TGF-B Signaling in Control of Cardiovascular Function
Downloaded from http://cshperspectives.cshlp.org/ on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press TGF-b Signaling in Control of Cardiovascular Function Marie-Jose´Goumans and Peter ten Dijke Department of Molecular Cell Biology and Cancer Genomics Centre Netherlands, Leiden University Medical Center, 2300 RC Leiden, The Netherlands Correspondence: [email protected] Genetic studies in animals and humans indicate that gene mutations that functionally perturb transforming growth factor b (TGF-b) signaling are linked to specific hereditary vascular syndromes, including Osler–Rendu–Weber disease or hereditary hemorrhagic telangiecta- sia and Marfan syndrome. Disturbed TGF-b signaling can also cause nonhereditary disorders like atherosclerosis and cardiac fibrosis. Accordingly, cell culture studies using endothelial cells or smooth muscle cells (SMCs), cultured alone or together in two- or three-dimensional cell culture assays, on plastic or embedded in matrix, have shown that TGF-b has a pivotal effect on endothelial and SMC proliferation, differentiation, migration, tube formation, and sprouting. Moreover, TGF-b can stimulate endothelial-to-mesenchymal transition, a process shown to be of key importance in heart valve cushion formation and in various pathological vascular processes. Here, we discuss the roles of TGF-b in vasculogenesis, angiogenesis, and lymphangiogenesis and the deregulation of TGF-b signaling in cardiovascular diseases. ransforming growth factor b1 (TGF-b1) is kawa et al. 2016). Consequently, many TGF-b Tthe prototype of a large family of structurally family cytokines play essential roles in embry- related, secreted dimeric proteins that have onic development, stem cells, and cell fate deter- pleiotropic effects and play important roles in mination and in adult tissue homeostasis and cell-to-cell signaling. -

23 Weeks Scan Copyright 2002 © by the Authors, ISUOG & Fetal Medicine Foundation, London
Contents Introduction 1 Standard views for examination of the fetus 2 Central nervous system Normal sonographic anatomy Neural tube defects Hydrocephalus and ventriculomegaly Holoprosencephaly Agenesis of the corpus collosum Dandy-Walker complex Microcephaly Megalencephaly Destructive cerebral lesions Arachnoid cysts Choroid plexus cysts Vein of Galen aneurysm 3 Face Normal sonographic anatomy Orbital defects Facial cleft Micrognathia 4 Cardiovascular system Introduction to congenital heart disease Assessment of the fetal heart Atrial septal defects Ventricular septal defects Atrioventricular septal defects Univentricular heart Aortic stenosis Coarctation and tubular hypoplasia of the aorta Interrupted aortic arch Hypoplastic left heart syndrome Pulmonary stenosis and pulmonary atresia Ebstein's anomaly and tricuspid valve dysplasia Conotruncal malformations Transposition of the great arteries Tetralogy of Fallot Double-outlet right ventricle Truncus arteriosus communis Cardiosplenic syndromes Echogenic foci Cardiac dysrhythmias: premature contractions Cardiac dysrhythmias: tachyarrhythmias Cardiac dysrhythmias: complete atrioventricular block 5 Pulmonary abnormalities Normal sonographic anatomy Cystic adenomatoid malformation Diaphragmatic hernia Pleural effusions Sequestration of the lungs 6 Anterior abdominal wall Normal sonographic anatomy Exomphalos Gastroschisis Body stalk anaomaly Bladder exstrophy and cloacal exstrophy 7 Gastrointestinal tract Normal sonographic anatomy Esopageal atresia Duodenal atresia Intestinal obstruction -

Atrioventricular Canal Defects
Cardiac Defects: Atrioventricular Canal Defects and the mitral valve the left. In a child with CAVC defect, there is one large valve that may not close correctly. As a result of the abnormal passageway between the two sides of the heart, blood from both sides mix and too much blood circulates back to the lungs before it travels through the body. This means the heart works harder than it should have to, and it will become enlarged and dam- aged if the problems aren’t repaired. Partial Atrioventricular Canal Defects A partial atrioventricular canal defect is the less severe form of this heart defect. The hole does not extend between the lower chambers of the heart and the valves are better formed. Usually it is necessary only to close the hole between the upper chambers (this hole is called an atrial septal defect, or ASD) and to do a minor repair of the mitral valve. Partial atrioventricular canal also is called atrioventricular septal defect (AVSD). ASD, atrial septal defect; VSD, ventricular septal defect. What are the symptoms of atrioventricular © The Children’s Hospital of Philadelphia. Reprinted with permission. canal defects? In CAVC defect, the following symptoms may be present within several days or weeks of birth: An atrioventricular canal defect is a problem in the part of • blue or purple tint to lips, skin, and nails (cyanosis) the heart that connects the upper chambers (atria) to the • difficulty breathing lower chambers (ventricles). There are two types of atrio- • poor weight gain and growth ventricular canal defects: complete and partial. • heart murmur—the heart sounds abnormal when a doctor listens with a stethoscope. -

Partial Atrioventricular Canal
Partial atrioventricular canal Author: Doctor Roberta Bini1 Creation Date: March 2003 Scientific Editor: Professor Bruno Marino 1Department of pediatric cardiology, Anna Meyer Hospital, Via Luca Giordano 13M, 50132 Firenze, Italy. [email protected] Abstract Keywords Disease name and synonyms Differential diagnosis Frequency Clinical description Diagnosis Natural history Treatment Etiology, genetic counselling and antenatal diagnosis Unresolved questions References Abstract Partial atrioventricular canal is due to a defective fusion of the endocardial cushions with the atrial septum primum. In the normal heart this fusion constitutes the atrioventricular septum where the mitral and tricuspid annuli insert, dividing the septum into an interatrial and an atrioventricular component. It accounts for about 4% of all congenital heart defects and its incidence is estimated to be 2 per 10,000 live births. It includes a spectrum of anomalies that differ from those of the complete form because the ventricular septal defect is absent. In partial atrioventricular canal two separate atrioventricular valve annuli are usually present: forms with a common annulus are referred as intermediate. Patients with these lesions may be asymptomatic or present with a variety of symptoms depending mostly on the function of the atrioventricular valves and the associated anomalies. Treatment of partial atrioventricular canal is invariably surgical and can be done electively in the first few years of life (uncomplicated forms) or in the first few months of life when symptoms are severe. Operative risk of all atrioventricular septal defects is approximately 3%. Twenty-year survival is 96%. Keywords Endocardial cushion defect, atrioventricular canal, atrioventricular septal defect, congenital heart disease Disease name and synonyms pulmonary hypertension with a complete form where the ventricular septal defect (VSD) may • Partial atrioventricular (AV) canal be obscured by the septal insertion of anterior • Ostium primum atrial septal defect and posterior common leaflets.