Mesentery — a 'New' Organ
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

BODY CAVITIES and MESENTERY
73: BODY CAVITIES and MESENTERY We've already mentioned that all the organs in the body are wrapped in "bags" made of thin layers of connective tissue. These bags are often inside of other bags, or even inside of several bags. The largest bags define areas that we call body cavities. There are three main cavities: the thoracic cavity, the abdominal cavity and the pelvic cavity. The thoracic cavity is subdivided into three smaller cavities: the pleural cavity (containing the lungs), the mediastinum(in the middle), and the pericardial cavity (containing the heart). The pleural cavity is easy to understand because it simply contains the lungs. The pericardial cavity contains not only the heart itself, but the large blood vessels that come out of it, such as the aorta. The pericardial cavity is inside of the third cavity, the mediastinum. ("Media" means "middle" and "stinum" can refer to the "sternum," which is the bone that runs down the center of the ribcage.) The mediastinum contains not only the pericardial cavity but also part of the esophagus and trachea, the thymus (remember this organ from module 2 on the immune system?), and quite a few nerves and lymph nodes. The thin layers of connective tissues that surround these cavities are made primarily of collagen and elastin (produced by fibroblast cells) but they also contain some very tiny nerves and blood vessels, as well as cells that make serous fluid. As we've seen in the past few lessons, the diaphragm separates the thoracic cavity from the abdominal cavity. The abdominal cavity contains the stomach, the spleen, the tail of the pancreas, the last half of the duodenum, the small intestines, most of the large intestines, and the mesentery (thin layers of connective tissue that anchor the intestines to the back wall of the abdominal cavity). -

Structure of the Human Body
STRUCTURE OF THE HUMAN BODY Vertebral Levels 2011 - 2012 Landmarks and internal structures found at various vertebral levels. Vertebral Landmark Internal Significance Level • Bifurcation of common carotid artery. C3 Hyoid bone Superior border of thyroid C4 cartilage • Larynx ends; trachea begins • Pharynx ends; esophagus begins • Inferior thyroid A crosses posterior to carotid sheath. • Middle cervical sympathetic ganglion C6 Cricoid cartilage behind inf. thyroid a. • Inferior laryngeal nerve enters the larynx. • Vertebral a. enters the transverse. Foramen of C 6. • Thoracic duct reaches its greatest height C7 Vertebra prominens • Isthmus of thyroid gland Sternoclavicular joint (it is a • Highest point of apex of lung. T1 finger's breadth below the bismuth of the thyroid gland T1-2 Superior angle of the scapula T2 Jugular notch T3 Base of spine of scapula • Division between superior and inferior mediastinum • Ascending aorta ends T4 Sternal angle (of Louis) • Arch of aorta begins & ends. • Trachea ends; primary bronchi begin • Heart T5-9 Body of sternum T7 Inferior angle of scapula • Inferior vena cava passes through T8 diaphragm T9 Xiphisternal junction • Costal slips of diaphragm T9-L3 Costal margin • Esophagus through diaphragm T10 • Aorta through diaphragm • Thoracic duct through diaphragm T12 • Azygos V. through diaphragm • Pyloris of stomach immediately above and to the right of the midline. • Duodenojejunal flexure to the left of midline and immediately below it Tran pyloric plane: Found at the • Pancreas on a line with it L1 midpoint between the jugular • Origin of Superior Mesenteric artery notch and the pubic symphysis • Hilum of kidneys: left is above and right is below. • Celiac a. -

Subserosal Haematoma of the Ileum
Arch Dis Child: first published as 10.1136/adc.35.183.509 on 1 October 1960. Downloaded from SUBSEROSAL HAEMATOMA OF THE ILEUM BY ANTONIO GENTIL MARTINS From the Department of Surgery, Alder Hey Children's Hospital, Liverpool (RECEIVED FCR PUBLICATION DECEMBER 21, 1959) Angiomas of the ileum are rare. Their association communicate with the lumen of the small bowel. with a duplication cyst has not so far been described. Opposite, the mucosa had a small erosion'. The unusual mode of presentation, with intestinal Microscopical examination (Figs. 3, 4 and 5) showed and a palpable mass (subserosal that 'considerable haemorrhage had occurred in the obstruction serous, muscular and mucous coats. The mucosa, haematoma) simulating intussusception, have however, was viable and the maximal zone of damage prompted the report of the present case. was towards the serosa. Numerous large capillaries were present in the coats. The lining of the diverticulum Case Report formed by glandular epithelium suggesting ileal mucosa N.C., a white male infant, born June 18, 1958, was was partly destroyed, but it had a well-formed muscular admitted to hospital on May 18, 1959, when 11 months coat': it was considered to be probably a duplication. old, with a five days' history of being irritable and appar- The main diagnosis was that of haemangioma of the ently suffering from severe colicky abdominal pain for ileum. the previous 24 hours. On the day of admission his bowels had not moved and he vomited several times. He looked pale and ill and a mass could be felt in the copyright. -
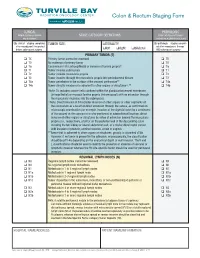
Colon & Rectum Staging Form
Colon & Rectum Staging Form CLINICAL PATHOLOGIC Extent of disease before STAGE CATEGORY DEFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical – staging completed TUMOR SIZE: LATERALITY: y pathologic – staging complet- after neoadjuvant therapy but ed after neoadjuvant therapy before subsequent surgery left right bilateral AND subsequent surgery PRIMARY TUMOR (T) TX Primary tumor cannot be assessed TX T0 No evidence of primary tumor T0 Tis Carcinoma in situ: intraepithelial or invasion of lamina propria* Tis T1 Tumor invades submucosa T1 T2 Tumor invades muscularis propria T2 T3 Tumor invades through the muscularis propria into pericolorectal tissues T3 T4a Tumor penetrates to the surface of the visceral peritoneum** T4a T4b Tumor directly invades or is adherent to other organs or structures^,** T4b *Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa. ^Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (for example, invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retro-peritoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix or vagina). **Tumor that is adherent to other organs or structures, grossly, is classified cT4b. -

Dynamic Contrast-Enhanced MRI Findings of Acute Pancreatitis in Ectopic Pancreatic Tissue: Case Report and Review of the Literature
University of Massachusetts Medical School eScholarship@UMMS Radiology Publications and Presentations Radiology 2014-07-28 Dynamic contrast-enhanced MRI findings of acute pancreatitis in ectopic pancreatic tissue: case report and review of the literature Senthur Thangasamy University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/radiology_pubs Part of the Digestive System Diseases Commons, and the Radiology Commons Repository Citation Thangasamy S, Zheng L, Mcintosh LJ, Lee P, Roychowdhury A. (2014). Dynamic contrast-enhanced MRI findings of acute pancreatitis in ectopic pancreatic tissue: case report and review of the literature. Radiology Publications and Presentations. https://doi.org/10.6092/1590-8577/2390. Retrieved from https://escholarship.umassmed.edu/radiology_pubs/259 Creative Commons License This work is licensed under a Creative Commons Attribution 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Radiology Publications and Presentations by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. JOP. J Pancreas (Online) 2014 July 28; 15(4):407-410 CASE REPORT Dynamic Contrast-Enhanced MRI Findings of Acute Pancreatitis in Ectopic Pancreatic Tissue: Case Report and Review of the Literature Senthur J Thangasamy1, Larry Zheng1, Lacey McIntosh1, Paul Lee2, Abhijit Roychowdhury1 1Department of Radiology and 2Pathology, University of Massachusetts Memorial Medical Center, Worcester, MA, USA ABSTRACT Context Acute pancreatitisCase report in ectopic pancreatic tissue is an uncommon cause of acute abdominal pain and can be difficult to diagnose on imaging. -

Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and Main Principals Of
Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and main principals of anatomical, pharmaceutical and clinical terminology (Student's book) Simferopol, 2017 Contents No. Topics Page 1. UNIT I. Latin language history. Phonetics. Alphabet. Vowels and consonants classification. Diphthongs. Digraphs. Letter combinations. 4-13 Syllable shortness and longitude. Stress rules. 2. UNIT II. Grammatical noun categories, declension characteristics, noun 14-25 dictionary forms, determination of the noun stems, nominative and genitive cases and their significance in terms formation. I-st noun declension. 3. UNIT III. Adjectives and its grammatical categories. Classes of adjectives. Adjective entries in dictionaries. Adjectives of the I-st group. Gender 26-36 endings, stem-determining. 4. UNIT IV. Adjectives of the 2-nd group. Morphological characteristics of two- and multi-word anatomical terms. Syntax of two- and multi-word 37-49 anatomical terms. Nouns of the 2nd declension 5. UNIT V. General characteristic of the nouns of the 3rd declension. Parisyllabic and imparisyllabic nouns. Types of stems of the nouns of the 50-58 3rd declension and their peculiarities. 3rd declension nouns in combination with agreed and non-agreed attributes 6. UNIT VI. Peculiarities of 3rd declension nouns of masculine, feminine and neuter genders. Muscle names referring to their functions. Exceptions to the 59-71 gender rule of 3rd declension nouns for all three genders 7. UNIT VII. 1st, 2nd and 3rd declension nouns in combination with II class adjectives. Present Participle and its declension. Anatomical terms 72-81 consisting of nouns and participles 8. UNIT VIII. Nouns of the 4th and 5th declensions and their combination with 82-89 adjectives 9. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Development of the Serosal Mesothelium
J. Dev. Biol. 2013, 1, 64-81; doi:10.3390/jdb1020064 OPEN ACCESS Journal of Developmental Biology ISSN 2221-3759 www.mdpi.com/journal/jdb Review Development of the Serosal Mesothelium Nichelle I. Winters and David M. Bader * Department of Medicine, Vanderbilt University, 2220 Pierce Ave Nashville, TN 37232, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-615-936-1976; Fax: +1-615-936-3527. Received: 3 May 2013; in revised form: 13 June 2013 / Accepted: 19 June 2013 / Published: 26 June 2013 Abstract: Mesothelia in the adult vertebrate are the simple squamous epithelia covering all coelomic organs and body cavities. Until recently, analysis of the generation and differentiative potential of mesothelia in organogenesis has largely focused on development of visceral mesothelium of the heart; the epicardium and its progenitor, the proepicardium. Here, we review emerging data on the development and differentiation of serosal mesothelium, the covering of the gastrointestinal tract. This literature demonstrates that serosal mesothelium is generated through a completely different mechanism than that seen in the heart suggesting that commitment of progenitors to this cell lineage does not follow a common pathway. The differentiative potential of serosal mesothelium is also discussed in comparison to that observed for progeny of the proepicardium/epicardium. In our review of the literature, we point out gaps in our understanding of serosal mesothelial development and that of mesothelial development as a whole. Keywords: mesothelium; proepicardium; epicardium; intestine; heart 1. Mesothelia: Broad Definition Mesothelia are simple squamous epithelia that line coelomic cavities and organs and form the mesenteries. -

TUMOR and TUMOR-LIKE CONDITIONS of the PERITONEUM and OMENTUM/MESENTERY 40Th
TUMOR AND TUMOR-LIKE CONDITIONS OF THE PERITONEUM AND OMENTUM/MESENTERY 40th. Annual Meeting SCBTMR September 9-13, 2017, Nashville, Tennessee Isaac R Francis University of Michigan Department of Radiology University of Michigan Disclosures • None • Acknowledgement: Dr. Ashish Wasnik, M.B.:B.S. Assistant Professor of Radiology University of Michigan PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Rare aggressive tumor • More common in men • Approx. 15% arise from peritoneum • Etiology: Exposure to asbestosis; other causes include mineral fiber exposure, radiation and chronic irritation • Three main histological subtypes: epithelioid (most common), sarcomatous (worst prognosis), and biphasic (mixed) • Frequent mutation of BRCA-Associated Protein (BAP)1 Levy AD et al Radiographics 2008; Alexander HR et al UptoDate 2016 PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Can present as focal, large or confluent peritoneal based mass/es (“dry” type) • Peritoneal nodular or diffuse thickening with ascites (“wet” type) • May show scalloping on adjacent abdominal organs- liver, spleen etc. • Calcification including calcified plaques is uncommon • Can infiltrate bowel mesentery, result in bowel wall thickening and may lead to bowel obstruction Levy AD et al Radiographics 2008 Peritoneal mesothelioma “Dry type” “Wet type” PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Cannot be easily distinguished from abdominal carcinomatosis • Absence of a known primary, absence of metastases to other organs or enlarged lymph nodes may help to distinguish -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

Mvdr. Natália Hvizdošová, Phd. Mudr. Zuzana Kováčová
MVDr. Natália Hvizdošová, PhD. MUDr. Zuzana Kováčová ABDOMEN Borders outer: xiphoid process, costal arch, Th12 iliac crest, anterior superior iliac spine (ASIS), inguinal lig., mons pubis internal: diaphragm (on the right side extends to the 4th intercostal space, on the left side extends to the 5th intercostal space) plane through terminal line Abdominal regions superior - epigastrium (regions: epigastric, hypochondriac left and right) middle - mesogastrium (regions: umbilical, lateral left and right) inferior - hypogastrium (regions: pubic, inguinal left and right) ABDOMINAL WALL Orientation lines xiphisternal line – Th8 subcostal line – L3 bispinal line (transtubercular) – L5 Clinically important lines transpyloric line – L1 (pylorus, duodenal bulb, fundus of gallbladder, superior mesenteric a., cisterna chyli, hilum of kidney, lower border of spinal cord) transumbilical line – L4 Bones Lumbar vertebrae (5): body vertebral arch – lamina of arch, pedicle of arch, superior and inferior vertebral notch – intervertebral foramen vertebral foramen spinous process superior articular process – mammillary process inferior articular process costal process – accessory process Sacrum base of sacrum – promontory, superior articular process lateral part – wing, auricular surface, sacral tuberosity pelvic surface – transverse lines (ridges), anterior sacral foramina dorsal surface – median, intermediate, lateral sacral crest, posterior sacral foramina, sacral horn, sacral canal, sacral hiatus apex of the sacrum Coccyx coccygeal horn Layers of the abdominal wall 1. SKIN 2. SUBCUTANEOUS TISSUE + SUPERFICIAL FASCIAS + SUPRAFASCIAL STRUCTURES Superficial fascias: Camper´s fascia (fatty layer) – downward becomes dartos m. Scarpa´s fascia (membranous layer) – downward becomes superficial perineal fascia of Colles´) dartos m. + Colles´ fascia = tunica dartos Suprafascial structures: Arteries and veins: cutaneous brr. of posterior intercostal a. and v., and musculophrenic a.