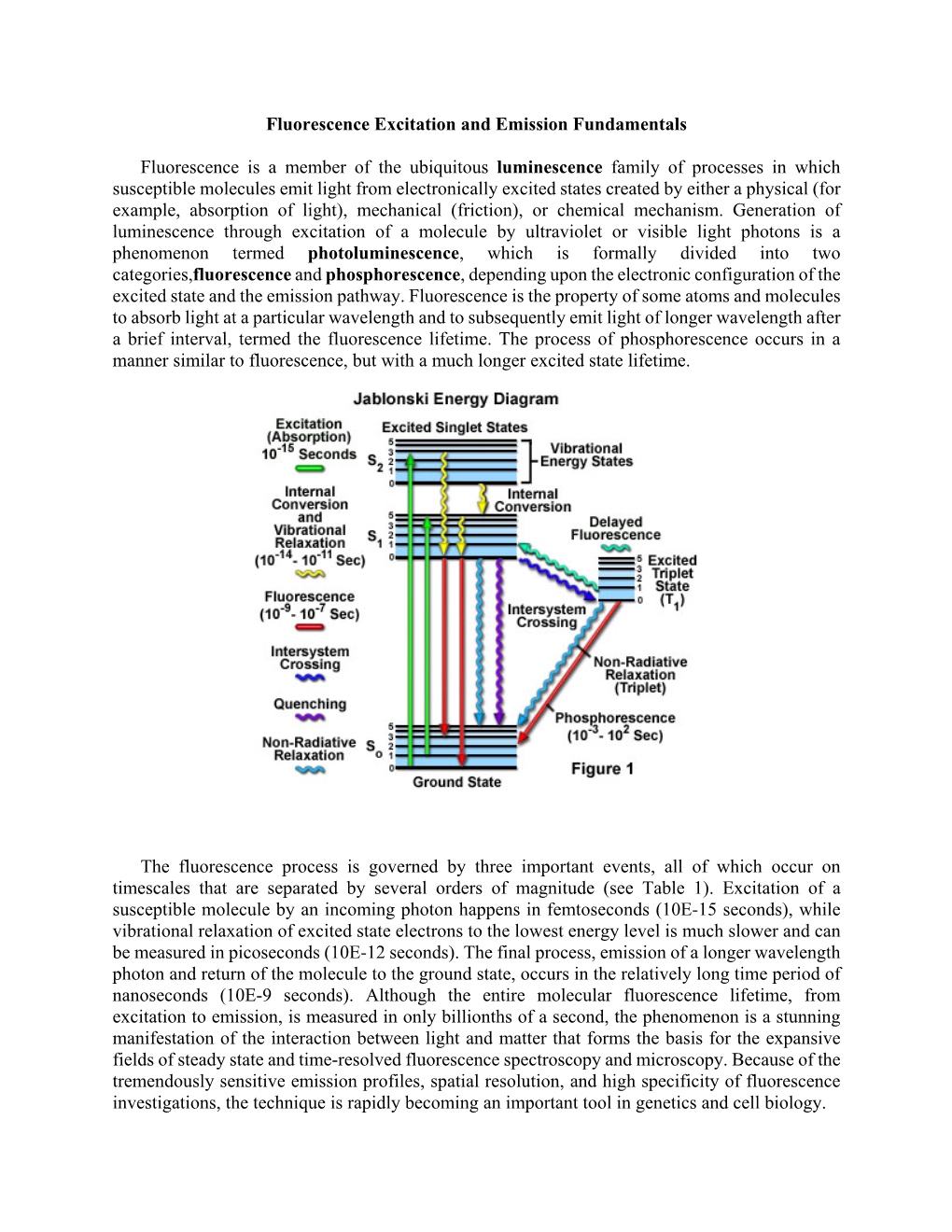
Fluorescence Excitation and Emission Fundamentals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Fluorescence Spectroscopy and Chemometrics in the Food Classification − a Review
Czech J. Food Sci. Vol. 25, No. 4: 159–173 Fluorescence Spectroscopy and Chemometrics in the Food Classification − a Review Jana SÁDECKÁ and Jana TÓTHOVÁ Institute of Analytical Chemistry, Faculty of Chemical and Food Technology, Slovak University of Technology, Bratislava, Slovak Republic Abstract Sádecká J., Tóthová J. (2007): Fluorescence spectroscopy and chemometrics in the food classification − a review. Czech J. Food Sci., 25: 159–173. This review deals with the last few years’ articles on various fluorescence techniques (conventional, excitation-emis- sion matrix, and synchronous fluorescence spectroscopy) as a tool for the classification of food samples. Chemometric methods as principal component analysis, hierarchical cluster analysis, parallel factor analysis, and factorial discrimi- nate analysis are briefly reminded. The respective publications are then listed according to the food samples: dairy products, eggs, meat, fish, edible oils, and others. Keywords: chemometrics; fluorescence spectroscopy; food analysis Fluorescence spectroscopy is a rapid, sensitive, distinguish. The analytical information contained and non-destructive analytical technique pro- in fluorescence spectra can be extracted by using viding in a few seconds spectral signatures that various multivariate analysis techniques that relate can be used as fingerprints of the food products several analytical variables to the properties of (dairy products, fishes, edible oils, wines, etc.). the analyte(s). The multivariate techniques most The application of fluorescence in food analysis frequently used allow to group the samples with has increased during the last decade, probably similar characteristics, to establish classification due to the propagated use of chemometrics. The methods for unknown samples (qualitative analysis) study by Norgaard (1995) can serve as a general or to perform methods determining some proper- investigation of how to enhance the potential of ties of unknown samples (quantitative analysis). -

Dozy-Chaos Mechanics for a Broad Audience
challenges Concept Paper Dozy-Chaos Mechanics for a Broad Audience Vladimir V. Egorov Russian Academy of Sciences, FSRC “Crystallography and Photonics”, Photochemistry Center, 7a Novatorov Street, 119421 Moscow, Russia; [email protected] Received: 29 June 2020; Accepted: 28 July 2020; Published: 31 July 2020 Abstract: A new and universal theoretical approach to the dynamics of the transient state in elementary physico-chemical processes, called dozy-chaos mechanics (Egorov, V.V. Heliyon Physics 2019, 5, e02579), is introduced to a wide general readership. Keywords: theoretical physics; quantum mechanics; molecular quantum transitions; singularity; dozy chaos; dozy-chaos mechanics; electron transfer; condensed matter; applications 1. Introduction In a publication of Petrenko and Stein [1], it is demonstrated that “the feasibility of the theory of extended multiphonon electron transitions” [2–4] “for the description of optical spectra of polymethine dyes and J-aggregates using quantum chemistry”. A recent publication by the same authors [5] “demonstrates the feasibility and applicability of the theory of extended multiphonon electron transitions for the description of nonlinear optical properties of polymethine dyes using quantum chemistry and model calculations”. About twenty years have passed since the first publications on the theory of extended multiphonon electron transitions [2–4], however, apart from the author of this theory and this paper, only Petrenko and Stein managed to use their results constructively, despite their promising character [1,5,6], and despite the fact that for a long time, the new theory has been constantly in the field of view of a sufficiently large number of researchers (see, e.g., [7–31]). The low demand in practice for the new, promising theory is associated not only with its complexity (however, we note that this theory is the simplest theory of all its likely future and more complex modifications [6]), but also with an insufficiently clear presentation of its physical foundations in the author’s pioneering works [2–4]. -

Comparison of Life-Cycle Analyses of Compact Fluorescent and Incandescent Lamps Based on Rated Life of Compact Fluorescent Lamp
Comparison of Life-Cycle Analyses of Compact Fluorescent and Incandescent Lamps Based on Rated Life of Compact Fluorescent Lamp Laurie Ramroth Rocky Mountain Institute February 2008 Image: Compact Fluorescent Lamp. From Mark Stozier on istockphoto. Abstract This paper addresses the debate over compact fluorescent lamps (CFLs) and incandescents through life-cycle analyses (LCA) conducted in the SimaPro1 life-cycle analysis program. It compares the environmental impacts of providing a given amount of light (approximately 1,600 lumens) from incandescents and CFLs for 10,000 hours. Special attention has been paid to recently raised concerns regarding CFLs—specifically that their complex manufacturing process uses so much energy that it outweighs the benefits of using CFLs, that turning CFLs on and off frequently eliminates their energy-efficiency benefits, and that they contain a large amount of mercury. The research shows that the efficiency benefits compensate for the added complexity in manufacturing, that while rapid on-off cycling of the lamp does reduce the environmental (and payback) benefits of CFLs they remain a net “win,” and that the mercury emitted over a CFL’s life—by power plants to power the CFL and by leakage on disposal—is still less than the mercury that can be attributed to powering the incandescent. RMI: Life Cycle of CFL and Incandescent 2 Heading Page Introduction................................................................................................................... 5 Background................................................................................................................... -

PHYSICS Glossary
Glossary High School Level PHYSICS Glossary English/Haitian TRANSLATION OF PHYSICS TERMS BASED ON THE COURSEWORK FOR REGENTS EXAMINATIONS IN PHYSICS WORD-FOR-WORD GLOSSARIES ARE USED FOR INSTRUCTION AND TESTING ACCOMMODATIONS FOR ELL/LEP STUDENTS THE STATE EDUCATION DEPARTMENT / THE UNIVERSITY OF THE STATE OF NEW YORK, ALBANY, NY 12234 NYS Language RBERN | English - Haitian PHYSICS Glossary | 2016 1 This Glossary belongs to (Student’s Name) High School / Class / Year __________________________________________________________ __________________________________________________________ __________________________________________________________ NYS Language RBERN | English - Haitian PHYSICS Glossary | 2016 2 Physics Glossary High School Level English / Haitian English Haitian A A aberration aberasyon ability kapasite absence absans absolute scale echèl absoli absolute zero zewo absoli absorption absòpsyon absorption spectrum espèk absòpsyon accelerate akselere acceleration akselerasyon acceleration of gravity akselerasyon pezantè accentuate aksantye, mete aksan sou accompany akonpaye accomplish akonpli, reyalize accordance akòdans, konkòdans account jistifye, eksplike accumulate akimile accuracy egzatitid accurate egzat, presi, fidèl achieve akonpli, reyalize acoustics akoustik action aksyon activity aktivite actual reyèl, vre addition adisyon adhesive adezif adjacent adjasan advantage avantaj NYS Language RBERN | English - Haitian PHYSICS Glossary | 2016 3 English Haitian aerodynamics ayewodinamik air pollution polisyon lè air resistance -

The Green Fluorescent Protein
P1: rpk/plb P2: rpk April 30, 1998 11:6 Annual Reviews AR057-17 Annu. Rev. Biochem. 1998. 67:509–44 Copyright c 1998 by Annual Reviews. All rights reserved THE GREEN FLUORESCENT PROTEIN Roger Y. Tsien Howard Hughes Medical Institute; University of California, San Diego; La Jolla, CA 92093-0647 KEY WORDS: Aequorea, mutants, chromophore, bioluminescence, GFP ABSTRACT In just three years, the green fluorescent protein (GFP) from the jellyfish Aequorea victoria has vaulted from obscurity to become one of the most widely studied and exploited proteins in biochemistry and cell biology. Its amazing ability to generate a highly visible, efficiently emitting internal fluorophore is both intrin- sically fascinating and tremendously valuable. High-resolution crystal structures of GFP offer unprecedented opportunities to understand and manipulate the rela- tion between protein structure and spectroscopic function. GFP has become well established as a marker of gene expression and protein targeting in intact cells and organisms. Mutagenesis and engineering of GFP into chimeric proteins are opening new vistas in physiological indicators, biosensors, and photochemical memories. CONTENTS NATURAL AND SCIENTIFIC HISTORY OF GFP .................................510 Discovery and Major Milestones .............................................510 Occurrence, Relation to Bioluminescence, and Comparison with Other Fluorescent Proteins .....................................511 PRIMARY, SECONDARY, TERTIARY, AND QUATERNARY STRUCTURE ...........512 Primary Sequence from -

Fluorescent Light-Emitting Diode (LED) Microscopy for Diagnosis of Tuberculosis
Fluorescent light-emitting diode (LED) microscopy for diagnosis of tuberculosis —Policy statement— March 2010 Contents Abbreviations Executive summary 1. Background 2. Evidence for policy formulation 2.1 Synthesis of evidence 2.2 Management of declarations of interest 3. Summary of results 4. Policy recommendations 5. Intended audience References Abbreviations CI confidence interval GRADE grades of recommendation assessment, development and evaluation LED light-emitting diode STAG-TB Strategic and Technical Advisory Group for Tuberculosis TB tuberculosis WHO World Health Organization Executive summary Conventional light microscopy of Ziehl-Neelsen-stained smears prepared directly from sputum specimens is the most widely available test for diagnosis of tuberculosis (TB) in resource-limited settings. Ziehl-Neelsen microscopy is highly specific, but its sensitivity is variable (20–80%) and is significantly reduced in patients with extrapulmonary TB and in HIV-infected TB patients. Conventional fluorescence microscopy is more sensitive than Ziehl-Neelsen and takes less time, but its use has been limited by the high cost of mercury vapour light sources, the need for regular maintenance and the requirement for a dark room. Light-emitting diodes (LED) have been developed to offer the benefits of fluorescence microscopy without the associated costs. In 2009, the evidence for the efficacy of LED microscopy was assessed by the World Health Organization (WHO), on the basis of standards appropriate for evaluating both the accuracy and the effect of new TB diagnostics on patients and public health. The results showed that the accuracy of LED microscopy was equivalent to that of international reference standards, it was more sensitive than conventional Ziehl-Neelsen microscopy and it had qualitative, operational and cost advantages over both conventional fluorescence and Ziehl- Neelsen microscopy. -

DEVELOPMENT of a RAMAN SPECTROMETER to STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K
BARC/2011/E/003 BARC/2011/E/003 DEVELOPMENT OF A RAMAN SPECTROMETER TO STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K. Sarkar and Tulsi Mukherjee Radiation & Photochemistry Division 2011 BARC/2011/E/003 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2011/E/003 DEVELOPMENT OF A RAMAN SPECTROMETER TO STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K. Sarkar and Tulsi Mukherjee Radiation & Photochemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2011 BARC/2011/E/003 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2011/E/003 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Development of a Raman spectrometer to study surface-enhanced Raman scattering 11 Collation : 31 p., 15 figs. 13 Project No. : 20 Personal author(s) : Nandita Biswas; Ridhima Chadha; Sudhir Kapoor; Sisir K. Sarkar; Tulsi Mukherjee 21 Affiliation of author(s) : Radiation and Photochemistry Division , Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Radiation and Photochemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2011/E/003 30 Date of submission : January 2011 31 Publication/Issue date : February 2011 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -

Xeu3+ Phosphors: X‑Ray Absorption and Emission Studies
www.nature.com/scientificreports OPEN Correlation among photoluminescence and the electronic and atomic 3+ structures of Sr2SiO4:xEu phosphors: X‑ray absorption and emission studies Shi‑Yan Zheng1,2, Jau‑Wern Chiou3*, Yueh‑Han Li3, Cheng‑Fu Yang4, Sekhar Chandra Ray5*, Kuan‑Hung Chen1, Chun‑Yu Chang1, Abhijeet R. Shelke1, Hsiao‑Tsu Wang1, Ping‑Hung Yeh1, Chun‑Yen Lai6, Shang‑Hsien Hsieh7, Chih‑Wen Pao7, Jeng‑Lung Chen7, Jyh‑Fu Lee7, Huang‑Ming Tsai7, Huang‑Wen Fu7, Chih‑Yu Hua7, Hong‑Ji Lin7, Chien‑Te Chen7 & Way‑Faung Pong1* 3+ 3+ 3+ A series of Eu ‑activated strontium silicate phosphors, Sr2SiO4:xEu (SSO:xEu , x = 1.0, 2.0 and 5.0%), were synthesized by a sol–gel method, and their crystalline structures, photoluminescence (PL) behaviors, electronic/atomic structures and bandgap properties were studied. The correlation among these characteristics was further established. X‑ray powder difraction analysis revealed the formation of mixed orthorhombic α’‑SSO and monoclinic β‑SSO phases of the SSO:xEu3+ phosphors. When SSO:xEu3+ phosphors are excited under ultraviolet (UV) light (λ = 250 nm, ~ 4.96 eV), they emit yellow (~ 590 nm), orange (~ 613 nm) and red (~ 652 and 703 nm) PL bands. These PL emissions 5 7 typically correspond to 4f–4f electronic transitions that involve the multiple excited D0 → FJ levels (J = 1, 2, 3 and 4) of Eu3+ activators in the host matrix. This mechanism of PL in the SSO:xEu3+ phosphors is strongly related to the local electronic/atomic structures of the Eu3+–O2− associations and the bandgap of the host lattice, as verifed by Sr K‑edge and Eu L3‑edge X‑ray absorption near‑edge structure (XANES)/extended X‑ray absorption fne structure, O K‑edge XANES and Kα X‑ray emission 3+ spectroscopy. -

Microenvironment-Triggered Dual-Activation of a Photosensitizer
www.nature.com/scientificreports OPEN Microenvironment‑triggered dual‑activation of a photosensitizer‑ fuorophore conjugate for tumor specifc imaging and photodynamic therapy Chang Wang1, Shengdan Wang1, Yuan Wang1, Honghai Wu1, Kun Bao2, Rong Sheng1* & Xin Li1* Photodynamic therapy is attracting increasing attention, but how to increase its tumor‑specifcity remains a daunting challenge. Herein we report a theranostic probe (azo‑pDT) that integrates pyropheophorbide α as a photosensitizer and a NIR fuorophore for tumor imaging. The two functionalities are linked with a hypoxic‑sensitive azo group. Under normal conditions, both the phototoxicity of the photosensitizer and the fuorescence of the fuorophore are inhibited. While under hypoxic condition, the reductive cleavage of the azo group will restore both functions, leading to tumor specifc fuorescence imaging and phototoxicity. The results showed that azo‑PDT selectively images BEL‑7402 cells under hypoxia, and simultaneously inhibits BEL‑7402 cell proliferation after near‑infrared irradiation under hypoxia, while little efect on BEL‑7402 cell viability was observed under normoxia. These results confrm the feasibility of our design strategy to improve the tumor‑ targeting ability of photodynamic therapy, and presents azo‑pDT probe as a promising dual functional agent. Cancer is one of the most common causes of death, and more and more therapeutic strategies against this fatal disease have emerged in the past few decades. Among these strategies, photodynamic therapy has attracted much attention1. Tis therapy is based on singlet oxygen produced by photosensitizers under the irradiation with light of a specifc wavelength to damage tumor tissues (Fig. 1a). Since the photo-damaging efect is induced by the interaction between a photosensitizer and light, tumor-specifc therapy may be realized by focusing the light to the tumor site. -
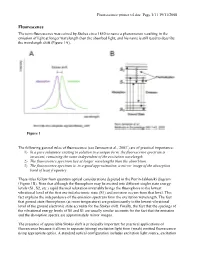
The Term Fluorescence Was Coined by Stokes Circa 1850 to Name A
Fluorescence primer v4.doc Page 1/11 19/11/2008 Fluorescence The term fluorescence was coined by Stokes circa 1850 to name a phenomenon resulting in the emission of light at longer wavelength than the absorbed light, and his name is still used to describe the wavelength shift (Figure 1A). Figure 1 The following general rules of fluorescence (see Jameson et al., 20031) are of practical importance: 1) In a pure substance existing in solution in a unique form, the fluorescence spectrum is invariant, remaining the same independent of the excitation wavelength. 2) The fluorescence spectrum lies at longer wavelengths than the absorbtion. 3) The fluorescence spectrum is, to a good approximation, a mirror image of the absorption band of least frequency These rules follow from quantum optical considerations depicted in the Perrin-Jablonski diagram (Figure 1B). Note that although the fluorophore may be excited into different singlet state energy levels (S1, S2, etc.) rapid thermal relaxation invariably brings the fluorophore to the lowest vibrational level of the first excited electronic state (S1) and emission occurs from that level. This fact explains the independence of the emission spectrum from the excitation wavelength. The fact that ground state fluorophores (at room temperature) are predominantly in the lowest vibrational level of the ground electronic state accounts for the Stokes shift. Finally, the fact that the spacings of the vibrational energy levels of S0 and S1 are usually similar accounts for the fact that the emission and the absorption spectra are approximately mirror images. The presence of appreciable Stokes shift is principally important for practical applications of fluorescence because it allows to separate (strong) excitation light from (weak) emitted fluorescence using appropriate optics. -

Resonance Raman Scattering of Light from a Diatomic Molecule
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Electrical & Computer Engineering, Department P. F. (Paul Frazer) Williams Publications of May 1976 Resonance Raman scattering of light from a diatomic molecule D. L. Rousseau Bell Laboratories, Murray Hill, New Jersey P. F. Williams University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/elecengwilliams Part of the Electrical and Computer Engineering Commons Rousseau, D. L. and Williams, P. F., "Resonance Raman scattering of light from a diatomic molecule" (1976). P. F. (Paul Frazer) Williams Publications. 24. https://digitalcommons.unl.edu/elecengwilliams/24 This Article is brought to you for free and open access by the Electrical & Computer Engineering, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in P. F. (Paul Frazer) Williams Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Resonance Raman scattering of light from a diatomic molecule D. L. Rousseau Bell Laboratories. Murray Hill, New Jersey 07974 P. F. Williams Bell Laboratories, Murray Hill, New Jersey 07974 and Department of Physics, University of Puerto Rico, Rio Piedras, Puerto Rico 00931 (Received 13 October 1975) Resonance Raman scattering from a homonuclear diatomic molecule is considered in detail. For convenience, the scattering may be classified into three excitation frequency regions-off-resonance Raman scattering for inciderit energies well away from resonance with any allowed transitions, discrete resonance Raman scattering for excitation near or in resonance with discrete transitions, and continuum resonance Raman scattering for excitation resonant with continuum transitions, e.g., excitation above a dissociation limit or into a repulsive electronic state.