Epidemiological Studies of Fructosamine in Relation to Diabetes, Cardiovascular Disease and Mortality
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
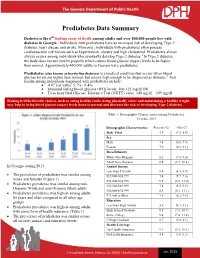
2015 Prediabetes Data Summary
The Georgia Department of Public Health Prediabetes Data Summary Diabetes is the 6th leading cause of death among adults and over 800,000 people live with diabetes in Georgia.1 Individuals with prediabetes have an increased risk of developing Type 2 diabetes, heart disease and stroke. Moreover, individuals with prediabetes often possess cardiovascular risk factors such as hypertension, obesity and high cholesterol. Prediabetes almost always occurs among individuals who eventually develop Type 2 diabetes.2 In Type 2 diabetes, the body does not use insulin properly which causes blood glucose (sugar) levels to be higher than normal. Approximately 450,000 adults in Georgia have prediabetes. Prediabetes (also known as borderline diabetes) is a medical condition that occurs when blood glucose levels are higher than normal, but are not high enough to be diagnosed as diabetes.3 Test results among individuals diagnosed with prediabetes include: A1C test value: 5.7% - 6.4% Impaired fasting blood glucose (IFG) levels: 100-125 mg/dl OR Two- hour Oral Glucose Tolerance Test (OGTT) value: 140 mg/dl – 199 mg/dl Making healt hy lifestyle choices, such as eating healthy foods, being physically active and maintaining a healthy weight, may help to bring blood glucose (sugar) levels down to normal and decrease the risk of developing Type 2 diabetes Table 1. Demographic Characteristics among Prediabetics, Georgia, 2013 Demographic Characteristics Percent (%) 95% CI State Total 7.9 (7.2, 8.9) Sex Male 7.8 (6.8, 9.5) Female 7.9 (6.9, 9.1) Race/Ethnicity -

CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E
Peer Reviewed PRACTICAL ONCOLOGY CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E. Burgess, DVM, Diplomate ACVIM (Oncology) Tufts University An insulinoma is a malignant pancreatic tumor that DIAGNOSIS inappropriately secretes excessive insulin, resulting in Aside from a histologic confirmation of insulinoma, profound hypoglycemia.1 no currently available diagnostic test provides a de- Pancreatic tumors are classified as: finitive diagnosis of insulinoma. Existing techniques • Exocrine, which includes adenocarcinomas of may help increase suspicion for an insulin-secreting ductular or acinar origin tumor but, with most diagnostic testing, it is im- • Endocrine, which arise from the islets of perative to interpret all results in the context of the Langerhans. coexisting clinical signs. Insulinomas are functional neuroendocrine tumors that originate in the beta cells of the islets Differential Diagnosis of Langerhans.1 A complete work-up, including careful patient history, physical examination, bloodwork, and PRESENTATION diagnostic imaging tests, should be performed to Signalment rule out other causes of hypoglycemia, such as Any breed of dog can be affected, but large sepsis, hepatic failure, adrenal cortical insufficiency, breeds tend to be overrepresented.1 While, in toxin ingestion, and other forms of neoplasia. humans, insulinomas affect females far more frequently than males, there is no apparent sex Laboratory Tests predilection in dogs.1-3 Dogs also commonly Blood Glucose present with a malignant variant, while humans A simple fasting blood glucose level of less than often have a benign adenoma (80%).1 Insulino- 40 mg/dL can suggest hyperinsulinemia, although ma is rare in cats.4 careful monitoring of a fasted dog with suspected insulinoma is strongly recommended due to high Clinical Signs risk for seizure activity. -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019
Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019 DEFINITION ⦁ Patient with known diabetes mellitus ⦁ Has a high blood sugar (hyperglycemia), defined as a blood glucose > 200 mg/dL (11 mmol/L) ⦁ Has symptoms of high blood sugar ⦁ Has questions regarding high blood sugar SYMPTOMS of High Blood Sugar (Hyperglycemia) include: ⦁ Mild hyperglycemia: Most often patient will have no symptoms. ⦁ Moderate hyperglycemia: polyuria, polydipsia, fatigue, blurred vision. ⦁ Severe hyperglycemia: confusion and coma. ⦁ Diabetic ketoacidosis (DKA): fruity odor on breath, vomiting, rapid breathing, weakness, confusion, and coma. INITIAL ASSESSMENT QUESTIONS 1. BLOOD GLUCOSE: "What is your blood glucose level?" 2. ONSET: "When did you check the blood glucose?" 3. USUAL RANGE: "What is your glucose level usually?" (e.g., usual fasting morning value, usual evening value) 4. KETONES: "Do you check for ketones (urine or blood test strips)?" If yes, ask: "What does the test show now?" 5. TYPE 1 or 2: "Do you know what type of diabetes you have?" (e.g., Type 1, Type 2, Gestational; doesn't know) 6. INSULIN: "Do you take insulin?" "What type of insulin(s) do you use? What is the mode of delivery? (syringe, pen (e.g., injection or pump) 7. DIABETES PILLS: "Do you take any pills for your diabetes?" If yes, ask: "Have you missed taking any pills recently?" 8. OTHER SYMPTOMS: "Do you have any symptoms?" (e.g., fever, frequent urination, difficulty breathing, dizziness, weakness, vomiting) 9. PREGNANCY: "Is there any chance you are pregnant?" -

Metformin for Prediabetes
FACT SHEET FOR PATIENTS AND FAMILIES Metformin for Prediabetes What is metformin? Metformin [met-FORE-min] is a medication that is often prescribed for prediabetes in order to help prevent type 2 diabetes. It is taken by mouth (orally) as a pill. Like other prediabetes medications, it works best when you follow the rest of your treatment plan, like improving your nutrition by eating more fruits and vegetables, being physically active every day, and in most cases, losing weight. What does it do? Metformin helps lower your blood glucose (blood sugar). It does this by: Does metformin cause hypoglycemia • Decreasing the amount of glucose released by your (low blood glucose)? liver. Less glucose enters into your bloodstream. No. Metformin doesn’t cause hypoglycemia by itself. • Increasing the ability of your muscles to use glucose But combined with other medications, vigorous for energy. As more glucose is used, more glucose exercise, or too little food, it can make your blood leaves your bloodstream. glucose drop too low. Why is metformin important Since low blood glucose can be dangerous, make sure for my health? that you and your family know the symptoms. These include feeling shaky, sweaty, hungry, and irritable. Metformin can’t cure your prediabetes. But, by If you have these symptoms, take some quick-acting helping control your blood glucose, it can help sugar. Good sources are 3 or 4 glucose tablets, a half- prevent type 2 diabetes, especially among those at cup of fruit juice or regular soda, or a tablespoon of highest risk for developing type 2 diabetes. -

Prediabetes: What Is It and What Can I Do?
Prediabetes: What Is It and What Can I Do? What is prediabetes? Weight loss can delay or prevent diabetes. Prediabetes is a condition that comes before Reaching a healthy weight can help you a lot. diabetes. It means your blood glucose levels are If you’re overweight, any weight loss, even higher than normal but aren’t high enough to be 7 percent of your weight (for example, losing called diabetes. about 15 pounds if you weigh 200), may lower your risk for diabetes. There are no clear symptoms of prediabetes. You can have it and not know it. If I have prediabetes, what does it mean? It means you might get type 2 diabetes soon or down the road. You are also more likely to get heart disease or have a stroke. The good news is that you can take steps to delay or prevent type 2 diabetes. How can I delay or prevent type 2 diabetes? You may be able to delay or prevent type 2 diabetes with: Daily physical activity, such as walking. Weight loss, if needed. Losing even a few pounds will help. Medication, if your doctor prescribes it. If you have prediabetes, these steps may bring your blood glucose to a normal range. But you are still at a higher risk for type 2 diabetes. Regular physical activity can delay or prevent diabetes. Being active is one of the best ways to delay or prevent type 2 diabetes. It can also lower your weight and blood pressure, and improve cholesterol levels. Ask your health care team about activities that are safe for you. -

What Everyone Needs to Know About Prediabetes
diaTribe.org provides free cutting-edge diabetes insights and actionable tips for people with diabetes. Our mission is to help individuals better understand their diabetes and to make our readers happier & healthier. What Everyone Needs to Know about Prediabetes: Symptoms, Diagnosis, Prevention, and more! What is prediabetes? Prediabetes is a condition where blood sugar levels are higher than normal, but not high enough to be diagnosed as type 2 diabetes. This occurs when the body has problems in processing glucose properly, and sugar starts to build up in the bloodstream instead of fueling cells in muscles and tissues. Insulin is the hormone that tells cells to take up glucose, and in prediabetes, people typically initially develop insulin resistance (where the body’s cells can’t respond to insulin as well), and over time the ability to produce sufficient insulin is reduced (if no actions are taken to reverse the situation). People with prediabetes also commonly have high blood pressure as well as abnormal blood lipids (e.g. cholesterol.) These often occur prior to the rise of blood glucose levels. What are the symptoms of prediabetes? People typically do not have symptoms of prediabetes, which is partially why up to 90% of people don’t know they have it. The ADA reports that some people with prediabetes may develop symptoms of type 2 diabetes, though even many people diagnosed with type 2 diabetes show little or no symptoms initially at diagnosis. These symptoms include: Urinating often Blurry vision Feeling very thirsty Feeling very hungry, even Cuts/bruises that Tingling, pain, or though you are eating are slow to heal numbness in the hands/ feet Extreme fatigue /diaTribeNews @diaTribeNews diaTribe.org “ People typically do not have symptoms of prediabetes, which is partially why up to 90% of people don’t know they have it.” Who is at risk of developing prediabetes? Overweight and obese adults (a BMI >25 kg/m2) are at significantly greater risk for developing prediabetes, as well as people with a family history of type 2 diabetes. -

Am I at Risk for Gestational Diabetes?
Am I at risk for gestational diabetes? U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH Eunice Kennedy Shriver National Institute of Child Health and Human Development What is gestational diabetes? Gestational diabetes (pronounced jess-TAY-shun-ul die-uh-BEET-eez) is a type of high blood sugar that only pregnant women get. In fact, the word “gestational” means pregnant. If a woman gets high blood sugar when she’s pregnant, but she never had high blood sugar before, she has gestational diabetes. Between 2 percent and 10 percent of U.S. pregnancies are affected by the condition every year,1 making it one of the top health concerns related to pregnancy. If not treated, gestational diabetes can cause problems for mothers and babies, some of them serious. But there is good news. Most of the time, gestational diabetes goes away after the baby is born. The changes in your body that cause gestational diabetes normally occur only when you are pregnant. After the baby is born, your body goes back to normal and the condition goes away. Gestational diabetes is treatable, and the best outcomes result from careful management and control of blood sugar levels. The best way to control gestational diabetes is to find out you have it early and start treatment quickly. Treating gestational diabetes—even if you don’t have any symptoms or your symptoms are mild— greatly reduces health problems for mother and baby. Why do some women get gestational diabetes? Usually, the body breaks down much of the food you eat into a type of sugar, called glucose (pronounced GLOO-kos). -

Assessing Blood Glucose Regulation
Functional Medicine University’s Functional Diagnostic Medicine Training Program Mod 4 * FDMT533B Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. Sodano, D.C., D.A.B.C.I., & Ron Grisanti, D.C., D.A.B.C.O., M.S. http://www.FunctionalMedicineUniversity.com Limits of Liability & Disclaimer of Warranty We have designed this book to provide information in regard to the subject matter covered. It is made available with the understanding that the authors are not liable for the misconceptions or misuse of information provided. The purpose of this book is to educate. It is not meant to be a comprehensive source for the topic covered, and is not intended as a substitute for medical diagnosis or treatment, or intended as a substitute for medical counseling. Information contained in this book should not be construed as a claim or representation that any treatment, process or interpretation mentioned constitutes a cure, palliative, or ameliorative. The information covered is intended to supplement the practitioner’s knowledge of their patient. It should be considered as adjunctive and support to other diagnostic medical procedures. This material contains elements protected under International and Federal Copyright laws and treaties. Any unauthorized reprint or use of this material is prohibited. Functional Medicine University; Functional Diagnostic Medicine Training Program/Insider’s Guide Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation Copyright © 2010 Functional Medicine University, All Rights Reserved Functional Medicine University’s Functional Diagnostic Medicine Training Program Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. -
Hyperglycemia – Prediabetes
North Dakota Family Planning Program Clinical Protocol Manual Health Maintenance – HM 8 Hyperglycemia – Prediabetes DEFINITION Hyperglycemia is an excess of glucose in the blood. Prediabetes is a condition when blood glucose levels are higher than normal but not yet high enough to be diagnosed as diabetes. (Prediabetes is also known as Impaired Glucose Tolerance (IGT) or Impaired Fasting Glucose (IFG) depending on which test was used to detect it. IGT uses the oral glucose tolerance test and IFG uses the fasting plasma glucose test.) SUBJECTIVE May include: 1. No symptoms 2. Family history of diabetes 3. Weight changes 4. Fatigue, blurred vision, recurrent vulvovaginal candidiasis 5. History of gestational diabetes or birth weight of newborn 9 lbs. or greater 6. Polydipsia, polyphagia, or polyuria 7. 20% over ideal body weight; sedentary lifestyle 8. Decreased wound healing 9. Race (higher incidence of diabetes among American Indian, Hispanic, and African American population) 10. History of hypertension 11. History of decreased HDL and increased triglycerides 12. History of Polycystic Ovarian Syndrome or Metabolic Syndrome 13. History of antipsychotic medication therapy OBJECTIVE May include: 1. Blood pressure 2. Height and weight 3. Complete physical exam. Acanthosis Nigricans may be noted in axillae, groin, and/or neck LABORATORY May refer for: 1. Hyperglycemia a. lab of choice may include one or more of the following: i. Fasting plasma glucose (FPG) a) Prediabetes: FPG between 100 mg/dl to 125 mg/dl b) Diabetes: FPG is > 126 mg/dl ii. Oral Glucose Tolerance Test (OGTT) a) Prediabetes: OGTT 140mg/dl to 199 mg/dl b) Diabetes: OGTT 200mg/dl or higher iii. -

Serum Fructosamine and Subsequent Breast Cancer Risk: a Nested Case-Control Study in the ORDET Prospective Cohort Study
Cancer Epidemiology, Biomarkers & Prevention 271 Short Communication Serum Fructosamine and Subsequent Breast Cancer Risk: A Nested Case-Control Study in the ORDET Prospective Cohort Study Mary Platek,1 Vittorio Krogh,6 Andrea Micheli,6 Richard Browne,5 Elisabetta Meneghini,6 Sabina Sieri,6 Holger J. Schu¨ nemann,2 Valeria Pala,6 Maddalena Barba,1 Gregory E. Wilding,3 Franco Berrino,6 and Paola Muti 4 Departments of 1Exercise and Nutrition Sciences, 2Medicine, 3Biostatistics, and 4Social and Preventive Medicine, 5Clinical Science Laboratory, University at Buffalo, State University of New York, Buffalo, New York; and 6Epidemiology Unit, Instituto Nazionale Per lo Studio e la Cura dei Tumori, Via Venezian, Milan, Italy Abstract There is evidence that abnormal glucose metabolism may follow-up, 144 breast cancer cases were identified and four contribute to the risk of breast cancer. The measurement of matched controls were selected from the cohort; serum markers of glucose metabolism could help to identify women fructosamine levels were measured in both groups at baseline. at risk for breast cancer. Serum fructosamine is one such Adjusted odds ratios (OR) for the highest tertile of serum marker. In this study, we investigated whether prediagnostic fructosamine compared to the lowest was 1.60 [95% con- serum fructosamine was associated with breast cancer. fidence interval (CI), 0.95-2.73]. In premenopausal women, the Between 1987 and 1992, 10,786 women ages 35 to 69 were OR was 1.58 (95% CI, 0.76-3.40) and in postmenopausal recruited in Italy for a prospective study. Women with a women, the OR was 1.60 (95% CI, 0.76-3.48). -

Fructosamine Interpretive Summary
Fructosamine Interpretive Summary Description: Fructosamine is a complex of glucose and protein that can be used to assess the average blood glucose concentration in a dog or cat over the previous 2-3 weeks. It is used in the diagnosis and management of diabetes mellitus. Decreased Fructosamine Common Causes Prolonged hypoglycemia o Insulin overdose o Insulinoma Decreased albumin (dog) or total protein (cat) Hyperthyroidism Uncommon Causes Increased serum triglycerides Azotemia Sample handling – storage at room temperature Related Findings Prolonged hypoglycemia o Insulinoma . Normal to increased serum insulin with concurrent decreased blood glucose . Increased insulin:glucose ratio . Pancreatic mass may be seen on ultrasound (cats>dogs) . A decreased fructosamine is not diagnostic for insulinoma but can increase clinical suspicion Hyperthyroidism o Increased T4, free T4 and free T4 by equilibrium dialysis Increased Fructosamine Common Causes Diabetes Mellitus Hemolysis (certain methodologies) Insulin overdose with Somogyi rebound effect Uncommon Causes Hypothyroidism Increased albumin (dog) or total protein (cat) Related Findings Diabetes Mellitus o Increased blood glucose o Glucose in urine +/- ketones Generated by VetConnect® PLUS: Fructosamine Page 1 of 2 Additional Information Physiology Fructosamine correlates with the patient’s average blood glucose concentration over the last 2-3 weeks. o Fructosamine is not affected by short-term increases in serum glucose such as those that occur with excitement, stress or intravenous dextrose administration. Fructosamine is a ketoamine that is formed by an irreversible, nonenzymatic linking of glucose to proteins (most often albumin and IgG). o Formation of fructosamine is related to the degree and duration of hyperglycemia. o Removal of fructosamine from the blood is dependent on the loss or degradation of the parent molecule (albumin).