Atomic Nuclei
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Computing ATOMIC NUCLEI
UNIVERSAL NUCLEAR ENERGY DENSITY FUNCTIONAL Computing ATOMIC NUCLEI Petascale computing helps disentangle the nuclear puzzle. The goal of the Universal Nuclear Energy Density Functional (UNEDF) collaboration is to provide a comprehensive description of all nuclei and their reactions based on the most accurate knowledge of the nuclear interaction, the most reliable theoretical approaches, and the massive use of computer power. Science of Nuclei the Hamiltonian matrix. Coupled cluster (CC) Nuclei comprise 99.9% of all baryonic matter in techniques, which were formulated by nuclear sci- the Universe and are the fuel that burns in stars. entists in the 1950s, are essential techniques in The rather complex nature of the nuclear forces chemistry today and have recently been resurgent among protons and neutrons generates a broad in nuclear structure. Quantum Monte Carlo tech- range and diversity in the nuclear phenomena that niques dominate studies of phase transitions in can be observed. As shown during the last decade, spin systems and nuclei. These methods are used developing a comprehensive description of all to understand both the nuclear and electronic nuclei and their reactions requires theoretical and equations of state in condensed systems, and they experimental investigations of rare isotopes with are used to investigate the excitation spectra in unusual neutron-to-proton ratios. These nuclei nuclei, atoms, and molecules. are labeled exotic, or rare, because they are not When applied to systems with many active par- typically found on Earth. They are difficult to pro- ticles, ab initio and configuration interaction duce experimentally because they usually have methods present computational challenges as the extremely short lifetimes. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

Nuclear Data
CNIC-00810 CNDC-0013 INDC(CPR)-031/L NUCLEAR DATA No. 10 (1993) China Nuclear Information Center Chinese Nuclear Data Center Atomic Energy Press CNIC-00810 CNDC-0013 INDC(CPR)-031/L COMMUNICATION OF NUCLEAR DATA PROGRESS No. 10 (1993) Chinese Nuclear Data Center China Nuclear Information Centre Atomic Energy Press Beijing, December 1993 EDITORIAL BOARD Editor-in-Chief Liu Tingjin Zhuang Youxiang Member Cai Chonghai Cai Dunjiu Chen Zhenpeng Huang Houkun Liu Tingjin Ma Gonggui Shen Qingbiao Tang Guoyou Tang Hongqing Wang Yansen Wang Yaoqing Zhang Jingshang Zhang Xianqing Zhuang Youxiang Editorial Department Li Manli Sun Naihong Li Shuzhen EDITORIAL NOTE This is the tenth issue of Communication of Nuclear Data Progress (CNDP), in which the achievements in nuclear data field since the last year in China are carried. It includes the measurements of 54Fe(d,a), 56Fe(d,2n), 58Ni(d,a), (d,an), (d,x)57Ni, 182~ 184W(d,2n), 186W(d,p), (d,2n) and S8Ni(n,a) reactions; theoretical calculations on n+160 and ,97Au, 10B(n,n) and (n,n') reac tions, channel theory of fission with diffusive dynamics; the evaluations of in termediate energy nuclear data for 56Fe, 63Cu, 65Cu(p,n) monitor reactions, and of 180Hf, ,8lTa(n,2n) reactions, revision on recommended data of 235U and 238U for CENDL-2; fission barrier parameters sublibrary, a PC software of EXFOR compilation, some reports on atomic and molecular data and covariance research. We hope that our readers and colleagues will not spare their comments, in order to improve the publication. Please write to Drs. -

CHEM 1412. Chapter 21. Nuclear Chemistry (Homework) Ky
CHEM 1412. Chapter 21. Nuclear Chemistry (Homework) Ky Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Consider the following statements about the nucleus. Which of these statements is false? a. The nucleus is a sizable fraction of the total volume of the atom. b. Neutrons and protons together constitute the nucleus. c. Nearly all the mass of an atom resides in the nucleus. d. The nuclei of all elements have approximately the same density. e. Electrons occupy essentially empty space around the nucleus. ____ 2. A term that is used to describe (only) different nuclear forms of the same element is: a. isotopes b. nucleons c. shells d. nuclei e. nuclides ____ 3. Which statement concerning stable nuclides and/or the "magic numbers" (such as 2, 8, 20, 28, 50, 82 or 128) is false? a. Nuclides with their number of neutrons equal to a "magic number" are especially stable. b. The existence of "magic numbers" suggests an energy level (shell) model for the nucleus. c. Nuclides with the sum of the numbers of their protons and neutrons equal to a "magic number" are especially stable. d. Above atomic number 20, the most stable nuclides have more protons than neutrons. e. Nuclides with their number of protons equal to a "magic number" are especially stable. ____ 4. The difference between the sum of the masses of the electrons, protons and neutrons of an atom (calculated mass) and the actual measured mass of the atom is called the ____. a. isotopic mass b. -

Nuclear Data Library for Incident Proton Energies to 150 Mev
LA-UR-00-1067 Approved for public release; distribution is unlimited. 7Li(p,n) Nuclear Data Library for Incident Proton Title: Energies to 150 MeV Author(s): S. G. Mashnik, M. B. Chadwick, P. G. Young, R. E. MacFarlane, and L. S. Waters Submitted to: http://lib-www.lanl.gov/la-pubs/00393814.pdf Los Alamos National Laboratory, an affirmative action/equal opportunity employer, is operated by the University of California for the U.S. Department of Energy under contract W-7405-ENG-36. By acceptance of this article, the publisher recognizes that the U.S. Government retains a nonexclusive, royalty- free license to publish or reproduce the published form of this contribution, or to allow others to do so, for U.S. Government purposes. Los Alamos National Laboratory requests that the publisher identify this article as work performed under the auspices of the U.S. Department of Energy. Los Alamos National Laboratory strongly supports academic freedom and a researcher's right to publish; as an institution, however, the Laboratory does not endorse the viewpoint of a publication or guarantee its technical correctness. FORM 836 (10/96) Li(p,n) Nuclear Data Library for Incident Proton Energies to 150 MeV S. G. Mashnik, M. B. Chadwick, P. G. Young, R. E. MacFarlane, and L. S. Waters Los Alamos National Laboratory, Los Alamos, NM 87545 Abstract Researchers at Los Alamos National Laboratory are considering the possibility of using the Low Energy Demonstration Accelerator (LEDA), constructed at LANSCE for the Ac- celerator Production of Tritium program (APT), as a neutron source. Evaluated nuclear data are needed for the p+ ¡ Li reaction, to predict neutron production from thin and thick lithium targets. -

Nuclear Energy Agency Nuclear Data Committee
NUCLEAR ENERGY AGENCY NUCLEAR DATA COMMITTEE SUMMARY RECORD OF THE lWEEFPY-FIRST MEETING (Technical Sessions) CBNM, Gee1 (Belgium) 24th-28th September 1979 Compiled by C. COCEVA (Scientific Secretary) OECD NUCLEAR ENERGY AGENCY 38 Bd. Suchet, 75016 Paris TABLE OF CONTENTS TECHNICAL SESSIONS Participants in meeting 1. Isotopes 2. National Progress Reports 3. Meetings 4. Technical Discussions 5. Topical Meeting on "Progress in Neutron Data of Structural Materials for Fast ~eactors" 6. Neutron and Related Nuclear Data Compilations and Evaluations Appendices 1 Meetings of the IAEA/NDS planned for 1980, 1981 and 1982 2 Progranme of the Topical Meeting on "Progress in Neutron Data of Structural Materials for Fast Reactors " 3 Summary of the general discussion on the works presented at the Topical Meeting TECmTICAL SESSIONS Perticipants in the 21st Meeting were as follows : For Canada : Dr. W.G. Cross Atomic Energy of Canada Ltd. Chalk River For Japan : Dr. K. Tsukada Japan Atomic Energy Research Institute Tokai-blur a For the United States of America : Dr. R.E. Chrien (Chairman) Brookhaven National Laboratory Dr. S.L. Wl~etstone U.S. Department of Energy Dr. 8.T. Motz Los Alamos Scientific Laboratory Dr. F.G. Perey Oak Ridge National Laboratory For the countries of the European Communities and the European Commission acting together : Dr. R. Iiockhoff (Local Secretary) Central Bureau for Nuclear Pleasurements Geel, Belgium Dr. C. Coceva (Scientific Secretary) Comitato Nazionale per 1'Energia Nucleare Bologna, Italy Dr. S. Cierjacks Kernforschungszentrum Karlsruhe Federal Republic of Germany Dr. C. Fort Conunissariat i 1'Energie Atomique Cadaroche, France Dr. A. Michaudon (Vice-chairman) Commissariat 2 1'Energie Atomique Bruysrcs-1.e-ChZtel Dr. -

The R-Process Nucleosynthesis and Related Challenges
EPJ Web of Conferences 165, 01025 (2017) DOI: 10.1051/epjconf/201716501025 NPA8 2017 The r-process nucleosynthesis and related challenges Stephane Goriely1,, Andreas Bauswein2, Hans-Thomas Janka3, Oliver Just4, and Else Pllumbi3 1Institut d’Astronomie et d’Astrophysique, Université Libre de Bruxelles, CP 226, 1050 Brussels, Belgium 2Heidelberger Institut fr¨ Theoretische Studien, Schloss-Wolfsbrunnenweg 35, 69118 Heidelberg, Germany 3Max-Planck-Institut für Astrophysik, Postfach 1317, 85741 Garching, Germany 4Astrophysical Big Bang Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan Abstract. The rapid neutron-capture process, or r-process, is known to be of fundamental importance for explaining the origin of approximately half of the A > 60 stable nuclei observed in nature. Recently, special attention has been paid to neutron star (NS) mergers following the confirmation by hydrodynamic simulations that a non-negligible amount of matter can be ejected and by nucleosynthesis calculations combined with the predicted astrophysical event rate that such a site can account for the majority of r-material in our Galaxy. We show here that the combined contribution of both the dynamical (prompt) ejecta expelled during binary NS or NS-black hole (BH) mergers and the neutrino and viscously driven outflows generated during the post-merger remnant evolution of relic BH-torus systems can lead to the production of r-process elements from mass number A > 90 up to actinides. The corresponding abundance distribution is found to reproduce the∼ solar distribution extremely well. It can also account for the elemental distributions observed in low-metallicity stars. However, major uncertainties still affect our under- standing of the composition of the ejected matter. -
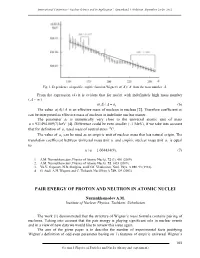
Pair Energy of Proton and Neutron in Atomic Nuclei
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2. -

1 Lecture Notes in Nuclear Structure Physics B. Alex Brown November
1 Lecture Notes in Nuclear Structure Physics B. Alex Brown November 2005 National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy Michigan State University, E. Lansing, MI 48824 CONTENTS 2 Contents 1 Nuclear masses 6 1.1 Masses and binding energies . 6 1.2 Q valuesandseparationenergies . 10 1.3 Theliquid-dropmodel .......................... 18 2 Rms charge radii 25 3 Charge densities and form factors 31 4 Overview of nuclear decays 40 4.1 Decaywidthsandlifetimes. 41 4.2 Alphaandclusterdecay ......................... 42 4.3 Betadecay................................. 51 4.3.1 BetadecayQvalues ....................... 52 4.3.2 Allowedbetadecay ........................ 53 4.3.3 Phase-space for allowed beta decay . 57 4.3.4 Weak-interaction coupling constants . 59 4.3.5 Doublebetadecay ........................ 59 4.4 Gammadecay............................... 61 4.4.1 Reduced transition probabilities for gamma decay . .... 62 4.4.2 Weisskopf units for gamma decay . 65 5 The Fermi gas model 68 6 Overview of the nuclear shell model 71 7 The one-body potential 77 7.1 Generalproperties ............................ 77 7.2 Theharmonic-oscillatorpotential . ... 79 7.3 Separation of intrinsic and center-of-mass motion . ....... 81 7.3.1 Thekineticenergy ........................ 81 7.3.2 Theharmonic-oscillator . 83 8 The Woods-Saxon potential 87 8.1 Generalform ............................... 87 8.2 Computer program for the Woods-Saxon potential . .... 91 8.2.1 Exampleforboundstates . 92 8.2.2 Changingthepotentialparameters . 93 8.2.3 Widthofanunboundstateresonance . 94 8.2.4 Width of an unbound state resonance at a fixed energy . 95 9 The general many-body problem for fermions 97 CONTENTS 3 10 Conserved quantum numbers 101 10.1 Angularmomentum. 101 10.2Parity .................................. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties.