Tetraacetic Acid (EGTA)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Feeding Acetyl-L-Carnitine and Lipoic Acid to Old Rats Significantly Improves Metabolic Function While Decreasing Oxidative Stress
Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress Tory M. Hagen*, Jiankang Liu†‡, Jens Lykkesfeldt§, Carol M. Wehr†, Russell T. Ingersoll‡, Vladimir Vinarsky†, James C. Bartholomew¶, and Bruce N. Ames†‡ʈ *Department of Biochemistry and Biophysics, Linus Pauling Institute, Oregon State University, Corvallis, OR 97331; †Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720; ‡Children’s Hospital Oakland Research Institute, Oakland, CA 94609; §Department of Pharmacology and Pathobiology, Royal Veterinary and Agricultural University, Copenhagen DK-1870, Denmark; and ¶Lawrence Berkeley National Laboratory, Berkeley, CA 94720 Contributed by Bruce N. Ames, December 28, 2001 Mitochondrial-supported bioenergetics decline and oxidative antioxidants or mitochondrial bioenergetics (13–15). Feeding stress increases during aging. To address whether the dietary old rats acetyl-L-carnitine (ALCAR), a mitochondrial metabo- addition of acetyl-L-carnitine [ALCAR, 1.5% (wt͞vol) in the drinking lite, reverses the age-related decline in tissue carnitine levels and water] and͞or (R)-␣-lipoic acid [LA, 0.5% (wt͞wt) in the chow] improves mitochondrial fatty acid -oxidation in the tissues improved these endpoints, young (2–4 mo) and old (24–28 mo) studied (15–18). ALCAR supplementation also reverses the F344 rats were supplemented for up to 1 mo before death and age-related alterations in fatty acid profiles and loss in cardio- ؉ hepatocyte isolation. ALCAR LA partially reversed the age-related lipin levels, an essential phospholipid required for mitochondrial decline in average mitochondrial membrane potential and signif- substrate transport (15–17). We demonstrated that ALCAR ؍ icantly increased (P 0.02) hepatocellular O2 consumption, indi- supplementation reverses the age-associated decline in meta- cating that mitochondrial-supported cellular metabolism was -؉ bolic activity in rats, suggesting that ALCAR improves mito markedly improved by this feeding regimen. -

Fe2+-Induced Lysis and Lipid Peroxidation of Chromaffin Granules
Journal of Neurochemisrry Raven Press, New York 0 1985 International Society for Neurochemistry Fe2’-Induced Lysis and Lipid Peroxidation of Chromaffin Granules Ronald M. Spears and Ronald W. Holz Department of Pharmacology, The University of Michigan Medical School, Ann Arbor, Michigan, U.S.A Abstract: Chromaffin granules, the catecholaminergic by trace amounts of reducible polyvalent cation. Lysis storage granules from adrenal chromaffin cells, lysed in sometimes occurred when Ca2+ was added with EGTA 10-9-10-7M Fez+. Lysis was accompanied by the pro- (10 pLM free Ca2+ concentration) and was consistently duction of malondialdehyde which results from lipid per- observed together with malondialdehyde production in oxidation. Both chromaffin granule lysis and malondi- the presence of Ca2+,EGTA, and 10 pM Fez+(total con- aldehyde production were inhibited by the free radical centration). The apparent Ca2+ dependency for chro- trapping agent butylated hydroxytoluene but not by cat- maffin granule lysis and malondialdehyde production was alase and/or superoxide dismutase. The results suggest probably caused by a trace reducible polyvalent ion dis- that lysis resulted from a direct transfer of electrons from placed by Ca2+from EGTA and not by a Ca2+-dependent Fe2+to a component of the chromaffin granule membrane reaction involving the chromaffin granule. Key Words: without the participation of either superoxide or hy- Chrornaffin granules- Fe2+- Malondialde hyde- Lipid drogen peroxide and may have resulted from lipid per- peroxidation-Ca2+. Spears R. M. and Holz R. W. oxidation. In some experiments, ascorbate alone induced Fe2+-induced lysis and lipid peroxidation of chromaffin chromaffin granule lysis which was inhibited by EDTA, granules. -

Deferiprone and Efonidipine Mitigated Iron-Overload Induced Neurotoxicity
Life Sciences 239 (2019) 116878 Contents lists available at ScienceDirect Life Sciences journal homepage: www.elsevier.com/locate/lifescie Deferiprone and efonidipine mitigated iron-overload induced neurotoxicity T in wild-type and thalassemic mice Jirapas Sripetchwandeea,b, Juthamas Khamseekaewb, Saovaros Svastic, Somdet Srichairatanakoold, Suthat Fucharoenc, Nipon Chattipakorna,b, ∗ Siriporn C. Chattipakorna,b,e, a Neurophysiology Unit, Cardiac Electrophysiology Research and Training Center, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand b Department of Physiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand c Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University, Nakhon Pathom, 73170, Thailand d Department of Biochemistry, Faculty of Medicine, Chiang Mai University, 50200, Thailand e Department of Oral Biology and Diagnostic Sciences, Faculty of Dentistry, Chiang Mai University, Chiang Mai, 50200, Thailand ARTICLE INFO ABSTRACT Keywords: Aims: We previously demonstrated that iron-overload in non-thalassemic rats induced neurotoxicity and cog- Iron-overload nitive decline. However, the effect of iron-overload on the brain of thalassemic condition has never beenin- Oxidative stress vestigated. An iron chelator (deferiprone) provides neuroprotective effects against metal toxicity. Furthermore, a Iron chelator T-type calcium channels blocker (efonidipine) effectively attenuates cardiac dysfunction in thalassemic mice T-type calcium channel blockers with iron-overload. However, the effects of both drugs on brain of iron-overload thalassemia has notbeen Mitochondria determined. We hypothesize that iron-overload induces neurotoxicity in Thalassemic and wild-type mice, and not only deferiprone, but also efonidipine, provides neuroprotection against iron-overload condition. Main methods: Mice from both wild-type (WT) and β-thalassemic type (HT) groups were assigned to be fed with a standard-diet or high-iron diet containing 0.2% ferrocene/kg of diet (HFe) for 4 months consecutively. -

Nitric Oxide Modulation of Interleukin-1Я-Evoked Intracellular
The Journal of Neuroscience, December 15, 2000, 20(24):8980–8986 Nitric Oxide Modulation of Interleukin-1-Evoked Intracellular Ca2؉ Release in Human Astrocytoma U-373 MG Cells and Brain Striatal Slices Antonella Meini,1 Alberto Benocci,1 Maria Frosini,1 Gianpietro Sgaragli,1 Gianpaolo Pessina,2 Carlo Aldinucci,2 Gise` le Tchuisseu Youmbi,1 and Mitri Palmi1 1Istituto di Scienze Farmacologiche and 2Istituto di Fisiologia, Universita` di Siena, 53100 Siena, Italy Intracellular Ca 2ϩ mobilization and release into mammal CSF Ca 2ϩ release induced by 2.5 but not 10 ng/ml IL-1. Ruthenium plays a fundamental role in the etiogenesis of fever induced by red (50 M) and, to a lesser extent, heparin (3 mg/ml) antagonized the proinflammatory cytokine interleukin-1 (IL-1) and other IL-1-induced Ca 2ϩ release, and both compounds administered pyrogens. The source and mechanism of IL-1-induced intracel- together completely abolished this response. Similar results were lular Ca 2ϩ mobilization was investigated using two experimental obtained in human astrocytoma cells in which IL-1 elicited a models. IL-1 (10 ng/ml) treatment of rat striatal slices preloaded delayed (30 min) increase in intracellular Ca 2ϩ concentration 45 2ϩ 2ϩ Ϯ with Ca elicited a delayed (30 min) and sustained increase ([Ca ]i ) (402 71.2% of baseline), which was abolished by 1 45 2ϩ (125–150%) in spontaneous Ca release that was potentiated mML-NAME. These data indicate that the NO/cGMP-signaling by L-arginine (300 M) and counteracted by N--nitro-L-arginine pathway is part of the intracellular mechanism transducing IL- 2ϩ methyl ester (L-NAME) (1 and 3 mM). -

(12) Patent Application Publication (10) Pub. No.: US 2014/0271461 A1 Reb Et Al
US 20140271461A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0271461 A1 Reb et al. (43) Pub. Date: Sep. 18, 2014 (54) COMPOSITIONS AND ASSOCATED Publication Classification METHODS FOR RADIOSOTOPE-BINDING MCROPARTICLES (51) Int. Cl. A615 L/2 (2006.01) (71) Applicant: Biosphere Medical, Inc., South Jordan, (52) U.S. Cl. UT (US) CPC .................................. A61K 51/1244 (2013.01) USPC ........................... 424/1.37: 525/360; 428/402 (72) Inventors: Philippe Reb, Themericourt (FR); Celine Chaix, Clichy la Garenne (FR) (57) ABSTRACT (73) Assignee: Biosphere Medical, Inc., South Jordan, The present disclosure relates to polymeric materials that UT (US) may be labeled with a radioisotope, to processes for produc ing the labeled polymeric material, and to methods of using (21) Appl. No.: 14/207,219 the materials in analytical and therapeutic applications. Spe cifically, the disclosure relates to injectable and implantable (22) Filed: Mar 12, 2014 microparticles. Such as microspheres, which are associated with radioisotopes such that the microparticles are both thera Related U.S. Application Data peutic and detectable. The radioisotope-containing micropar (60) Provisional application No. 61/779,712, filed on Mar. ticles are useful for embolization and other therapeutic medi 13, 2013. cal applications. Patent Application Publication Sep. 18, 2014 Sheet 1 of 11 US 2014/0271461 A1 FIG. 1 Patent Application Publication Sep. 18, 2014 Sheet 2 of 11 US 2014/0271461 A1 FIG. 2 Patent Application Publication Sep. 18, 2014 Sheet 3 of 11 US 2014/0271461 A1 FIG 3 Patent Application Publication Sep. 18, 2014 Sheet 4 of 11 US 2014/0271461 A1 FIG. -

A Mitochondria-Targeted S-Nitrosothiol Modulates Respiration, Nitrosates Thiols, and Protects Against Ischemia-Reperfusion Injury
A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury Tracy A. Primea, Frances H. Blaikieb, Cameron Evansb, Sergiy M. Nadtochiyc, Andrew M. Jamesa, Christina C. Dahma, Dario A. Vitturid, Rakesh P. Pateld, C. Robin Hileye, Irina Abakumovaa, Raquel Requejoa, Edward T. Chouchania, Thomas R. Hurda, John F. Garveyf, Cormac T. Taylorf, Paul S. Brookesc, Robin A. J. Smithb, and Michael P. Murphya,1 aMRC Mitochondrial Biology Unit, Hills Road, Cambridge CB2 0XY, United Kingdom; bDepartment of Chemistry, University of Otago, P. O. Box 56, Dunedin 9054, New Zealand; cDepartment of Anesthesiology, University of Rochester Medical Center, 601 Elmwood Avenue, Rochester, NY 14642; dDepartment of Pathology and Center for Free Radical Biology, University of Alabama at Birmingham, 901 19th Street South, Birmingham, AL 35294; eDepartment of Pharmacology, University of Cambridge, Tennis Court Road, Cambridge CB2 1PD, United Kingdom; and fConway Institute, University College, Dublin, Dublin 4, Ireland Edited by Salvador Moncada, University College London, London, United Kingdom, and approved May 1, 2009 (received for review March 25, 2009) Nitric oxide (NO•) competitively inhibits oxygen consumption by phonium (TPP) cationic moiety, which has been used to target mitochondria at cytochrome c oxidase and S-nitrosates thiol pro- molecules to mitochondria both in vitro and in vivo (11). The teins. We developed mitochondria-targeted S-nitrosothiols (MitoS- delocalized positive charge and lipophilic surface enables TPP NOs) that selectively modulate and protect mitochondrial function. cations to cross biological membranes rapidly without a require- The exemplar MitoSNO1, produced by covalently linking an S- ment for a carrier protein, and to accumulate several hundred- nitrosothiol to the lipophilic triphenylphosphonium cation, was fold in energized mitochondria in vivo driven by the membrane rapidly and extensively accumulated within mitochondria, driven potential (⌬) (12). -

United States Patent (10) Patent No.: US 9,687,573 B2 Reb Et Al
US009687573B2 (12) United States Patent (10) Patent No.: US 9,687,573 B2 Reb et al. (45) Date of Patent: Jun. 27, 2017 (54) COMPOSITIONS AND ASSOCIATED 5,762.903. A 6/1998 Parket al. METHODS FOR RADIOSOTOPE-BINDING 3. A 3. E. et al. e MCROPARTICLES 5,885,547 A 3/1999 Gray 5,888,546 A 3, 1999 Ji et al. (71) Applicant: Biosphere Medical, Inc., South Jordan, 5,894,022 A 4/1999 Ji et al. UT (US) 5,942,209 A 8, 1999 Leavitt et al. 6,015,541 A 1/2000 Greff et al. (72) Inventors: Philippe Reb, Themericourt (FR): 6,039,970 A 3/2000 Callegaro et al. Celine Chaix, Clichy la Garenne (FR) 6,060,040 A * 5/2000 Tournier ................ A61K424/9.364 49,06 6,083,495 A 7/2000 Holmes-Farley et al. (73) Assignee: Biosphere Medical, Inc., South Jordan, 6,099.457 A 8, 2000 Good UT (US) 6,103,295 A 8, 2000 Chan et al. 6,106,454. A 8/2000 Berg et al. (*) Notice: Subject to any disclaimer, the term of this 6,168,777 B1 1/2001 Greff et al. patent is extended or adjusted under 35 4.2.95. r. I R $39. St.reff et al. U.S.C. 154(b) by 0 days. 6,231,615 B1 5/2001 Preissman 6,241,962 B1 6/2001 Nicolini (21) Appl. No.: 14/207,219 6.248,057 B1 6/2001 Mavity et al. 6,258.338 B1 7/2001 Gray (22) Filed: Mar 12, 2014 6,273,851 B1 8, 2001 Slater et al. -

D-Penicillamine Cooperates with Copper Sulfate to Enhance the Surface Expression of Functional Fas Antigen in Rheumatoid Synovia
D-penicillamine cooperates with copper sulfate to enhance the surface expression of functional Fas antigen in rheumatoid synovial fibroblasts via the generation of hydrogen peroxide S. Harada, E. Sugiyama, H. Taki, K. Shinoda, T. Fujita, M. Maruyama, M. Kobayashi First Department of Internal Medicine, Toyama Medical and Pharmaceutical University, Toyama, Japan. Abstract Objective D-penicillamine (DP) has been shown to cooperate with copper ion to inhibit cell growth in a variety of cell types. To determine whether this inhibitory action is involved in Fas-mediated apoptosis, we examined the effect of DP and copper sulfate on the expression and function of Fas antigen in rheumatoid synovial fibroblasts (RSFs). Methods The expression of Fas antigen on the cell surface was determined by flow cytometric analysis. Western blot analysis was performed to examine the protein expressions of Fas and Fas-ligand. In addition, the amounts of apoptotic cells were determined by 4’, 6-diamidino-2’-phenylindol dihydrochloride (DAPI) and propidium iodide (PI) staining. Results Although DP and copper sulfate alone did not affect the surface expression of Fas antigen on RSFs, both in combination augmented the Fas expression in dose- and time-dependent manners. The enhanced expression of Fas antigen on their surface was also observed in interleukin-1 (IL-1 ) and/or tumor necrosis factor (TNF ) stimulated RSFs. On the other hand, the combination of DP and copper sulfate did not increase the amounts of cellular Fas protein, as determined by Western blot analysis. To determine whether the induced Fas antigen is functional, we examined the effect of DP and copper sulfate on Fas-mediated apoptosis, using an agonistic anti- Fas antibody. -

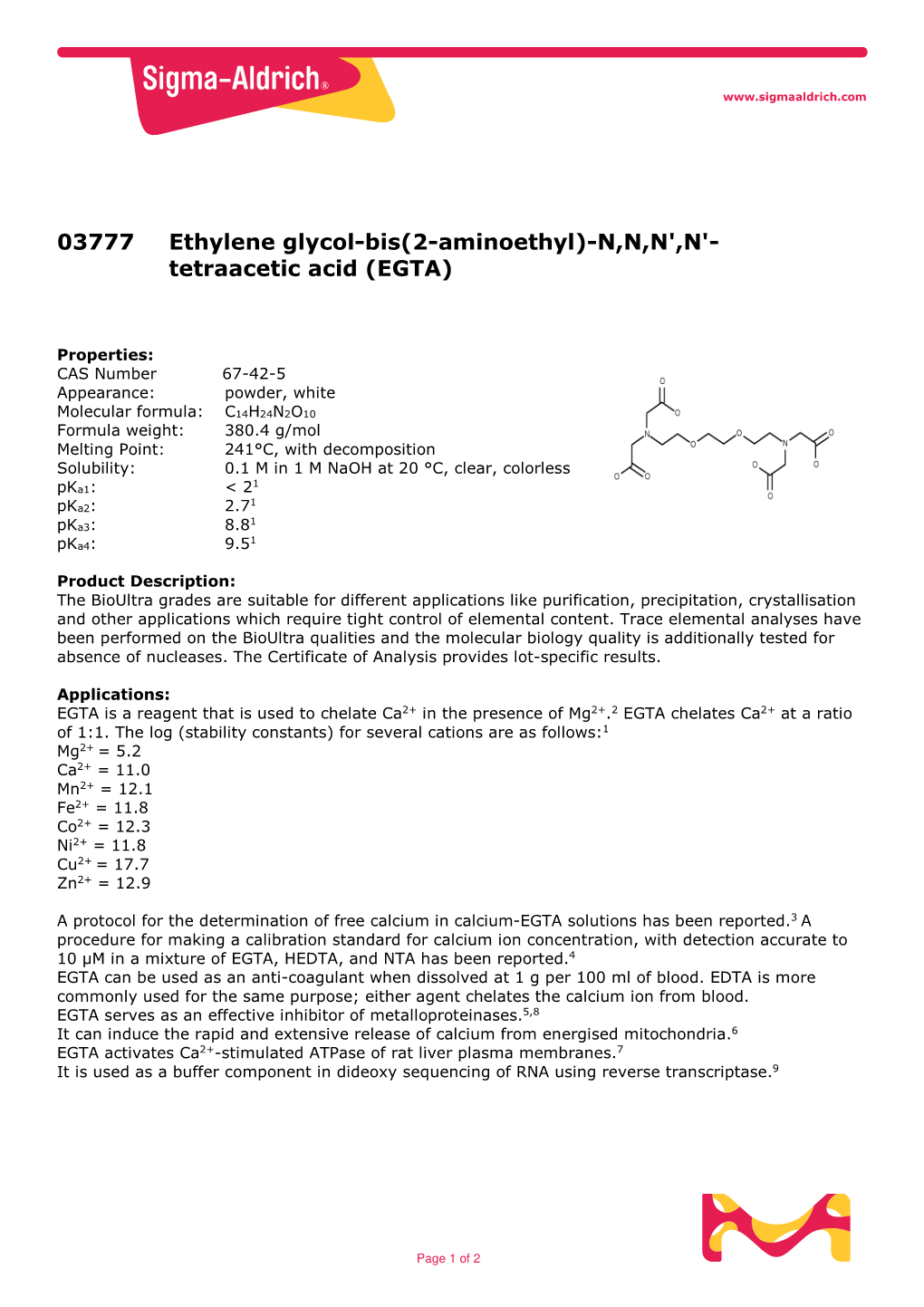
Tetraacetic Acid Product Number E3889 Store at Room Temperature
Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid Product Number E3889 Store at Room Temperature Product Description Precautions and Disclaimer Molecular Formula: C14H24N2O10 For Laboratory Use Only. Not for drug, household or Molecular Weight: 380.4 other uses. CAS Number: 67-42-5 1 pKa: < 2, 2.7, 8.8, and 9.5 Preparation Instructions Melting Point: 241 °C, with decomposition This product is soluble in 1 M NaOH (110 mg/ml). A Synonym: EGTA saturated solution at room temperature was found to be 2 mM in EGTA and had a pH of 2.72. This product This product is designated as Molecular Biology grade has the following maximal solubilities in aqueous and is suitable for molecular biology applications. It media at the respective pH values: has been analyzed for the presence of nucleases and proteases. pH 8.4 > 0.52 M pH 5.4 > 0.48 M EGTA is a reagent that is used to chelate Ca2+ in the pH 4.5 = 0.45 M presence of Mg2+.2 pH 4.2 = 0.42 M EGTA chelates Ca2+ at a ratio of 1:1. The log (stability pH 4.0 = 0.31 M constants) for several cations are as follows:1 References Mg2+ = 5.2 1. Data for Biochemical Research, 3rd ed., Dawson, Ca2+ = 11.0 R.M.C., et al., Oxford University Press (New York, Mn2+ = 12.1 NY: 1986), pp. 404-405. Fe2+ = 11.8 2. Schmid, R.W., and Reilley, C.N., New complexon Co2+ = 12.3 for titration of calcium in the presence of Ni2+ = 11.8 magnesium. -

Table of Contents
MONTANA TECH i CHEMICAL HYGIENE PLAN TABLE OF CONTENTS TABLE OF CONTENTS .......................................................................................... i STATEMENT OF POLICY .................................................................................... 1 CHEMICAL HYGIENE RESPONSIBILITIES ........................................................ 2 LABORATORY FACILITIES ................................................................................. 4 Maintenance ..................................................................................................... 4 Evaluation ......................................................................................................... 5 CONTROL MEASURES ....................................................................................... 5 Work Habits ...................................................................................................... 5 Laboratory Hygiene ........................................................................................... 6 Engineering Controls ........................................................................................ 6 Instrument Equipment and Use ........................................................................ 7 Personal Protective Equipment (PPE) .............................................................. 8 Eye Protection ............................................................................................. 8 Hand Protection .......................................................................................... -
(12) Patent Application Publication (10) Pub. No.: US 2010/0249747 A1 Mills Et Al
US 2010O24.9747A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0249747 A1 Mills et al. (43) Pub. Date: Sep. 30, 2010 (54) TRANSDERMAL VENOUS ACCESS Publication Classification LOCKING SOLUTION (51) Int. Cl. A6M 25/00 (2006.01) (75) Inventors: Stanley L. Mills, Goldsby, OK A 6LX 3L/727 (2006.01) (US); Jacqueline L. Mills, A638/17 (2006.01) Goldsby, OK (US); Robert D. A6IP 7/02 (2006.01) Maurer, Elkhorn, NE (US); Gary A6II 3L/20 (2006.01) L. Rayburn, Norman, OK (US); AOIN 37/00 (2006.01) Marvin A. Cuchens, Madison, MS A6IP3L/00 (2006.01) (US) (52) U.S. Cl. ............. 604/506; 514/56; 514/12: 514/558; 514/557; 604/265 Correspondence Address: (57) ABSTRACT Richard G. Gervase Mintz Levin Cohn Ferris Glovsky and Popeo PC Compositions and methods of employing compositions in Chrysler Center, 666 Third Avenue, 24th Floor flushing and coating medical devices are disclosed. The com New York, NY 10017 (US) positions include combinations of a chelating agent, antico agulant, or antithrombotic agent, with a C-C carboxylate antimicrobial agent, such as octanoic acid. Methods of using (73) Assignee: Organic Medical Ventures, these compositions for coating a medical device and for L.L.C., Norman, OK (US) inhibiting catheter infection are also disclosed. Particular combinations of the claimed combinations include, for (21) Appl. No.: 12/383,722 example, octanoic acid or other C-C carboxylate antimicro bial agent together with EDTA, EGTA, DTPA, heparin and/or (22) Filed: Mar. 26, 2009 hirudin in a pharmaceutically acceptable diluent. SOLUTIONY-...-- Patent Application Publication Sep. -

Downloaded from Bioscientifica.Com at 09/27/2021 05:05:07PM Via Free Access 66 T
Reproduction (2002) 124, 65–71 Research Alleviation of the two-cell block of ICR mouse embryos by polyaminocarboxylate metal chelators T. Matsukawa, S. Ikeda, H. Imai and M. Yamada* Laboratory of Reproductive Physiology, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan The present study was undertaken to examine the with that (< 25%) in medium without polyamino- effects of various transition metal ion chelators, both poly- carboxylates. Although EDDA, EDTA and DTPA at aminocarboxylates (including nitrilotriacetate (NTA), 10 µmol l–1 induced the development of most one-cell ethylenediaminediacetate (EDDA), ethyleneglycolbistetra- embryos to the four-cell stage and beyond, a higher acetate (EGTA), ethylenediaminetetraacetate (EDTA) and concentration (100 µmol l–1) of NTA and EGTA was diethylenetriaminepentaacetate (DTPA)) and non- required to obtain a similar result. Therefore, the ability of polyaminocarboxylates (dipicolinic acid and deferoxamine), polyaminocarboxylates to overcome the two-cell block is on the development in vitro of one-cell ICR strain mouse not correlated with their potency to chelate transition embryos to the four-cell and blastocyst stages. The order of metal ions. In contrast, the non-polyaminocarboxylates stability constants of polyaminocarboxylates for transition dipicolinic acid and deferoxamine, at 10 and 100 µmol l–1, metal ions such as zinc, copper and iron is as follows: did not have the same effect. Taken together, the results NTA р EDDA < EGTA < EDTA < DTPA. Addition of 10 indicate that the ability of polyaminocarboxylates to or 100 µmol polyaminocarboxylates l–1 to the medium overcome the two-cell block in embryo development is significantly enhanced the development of most one-cell due to some common feature or features other than the embryos (66–88%) beyond the two-cell stage compared ability to chelate transition metal ions.