Avian Diversity and Habitat Use on Wetland Reserve Program Lands in the Lower Missouri River Valley
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Flooding the Missouri Valley the Politics of Dam Site Selection and Design
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Great Plains Quarterly Great Plains Studies, Center for Summer 1997 Flooding The Missouri Valley The Politics Of Dam Site Selection And Design Robert Kelley Schneiders Texas Tech University Follow this and additional works at: https://digitalcommons.unl.edu/greatplainsquarterly Part of the Other International and Area Studies Commons Schneiders, Robert Kelley, "Flooding The Missouri Valley The Politics Of Dam Site Selection And Design" (1997). Great Plains Quarterly. 1954. https://digitalcommons.unl.edu/greatplainsquarterly/1954 This Article is brought to you for free and open access by the Great Plains Studies, Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Great Plains Quarterly by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. FLOODING THE MISSOURI VALLEY THE POLITICS OF DAM SITE SELECTION AND DESIGN ROBERT KELLEY SCHNEIDERS In December 1944 the United States Con Dakota is 160 feet high and 10,700 feet long. gress passed a Rivers and Harbors Bill that The reservoir behind it stretches 140 miles authorized the construction of the Pick-Sloan north-northwest along the Missouri Valley. plan for Missouri River development. From Oahe Dam, near Pierre, South Dakota, sur 1946 to 1966, the United States Army Corps passes even Fort Randall Dam at 242 feet high of Engineers, with the assistance of private and 9300 feet long.! Oahe's reservoir stretches contractors, implemented much of that plan 250 miles upstream. The completion of Gar in the Missouri River Valley. In that twenty rison Dam in North Dakota, and Oahe, Big year period, five of the world's largest earthen Bend, Fort Randall, and Gavin's Point dams dams were built across the main-stem of the in South Dakota resulted in the innundation Missouri River in North and South Dakota. -
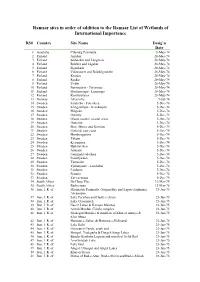
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Missouri River Floodplain from River Mile (RM) 670 South of Decatur, Nebraska to RM 0 at St
Hydrogeomorphic Evaluation of Ecosystem Restoration Options For The Missouri River Floodplain From River Mile (RM) 670 South of Decatur, Nebraska to RM 0 at St. Louis, Missouri Prepared For: U. S. Fish and Wildlife Service Region 3 Minneapolis, Minnesota Greenbrier Wetland Services Report 15-02 Mickey E. Heitmeyer Joseph L. Bartletti Josh D. Eash December 2015 HYDROGEOMORPHIC EVALUATION OF ECOSYSTEM RESTORATION OPTIONS FOR THE MISSOURI RIVER FLOODPLAIN FROM RIVER MILE (RM) 670 SOUTH OF DECATUR, NEBRASKA TO RM 0 AT ST. LOUIS, MISSOURI Prepared For: U. S. Fish and Wildlife Service Region 3 Refuges and Wildlife Minneapolis, Minnesota By: Mickey E. Heitmeyer Greenbrier Wetland Services Advance, MO 63730 Joseph L. Bartletti Prairie Engineers of Illinois, P.C. Springfield, IL 62703 And Josh D. Eash U.S. Fish and Wildlife Service, Region 3 Water Resources Branch Bloomington, MN 55437 Greenbrier Wetland Services Report No. 15-02 December 2015 Mickey E. Heitmeyer, PhD Greenbrier Wetland Services Route 2, Box 2735 Advance, MO 63730 www.GreenbrierWetland.com Publication No. 15-02 Suggested citation: Heitmeyer, M. E., J. L. Bartletti, and J. D. Eash. 2015. Hydrogeomorphic evaluation of ecosystem restoration options for the Missouri River Flood- plain from River Mile (RM) 670 south of Decatur, Nebraska to RM 0 at St. Louis, Missouri. Prepared for U. S. Fish and Wildlife Service Region 3, Min- neapolis, MN. Greenbrier Wetland Services Report 15-02, Blue Heron Conservation Design and Print- ing LLC, Bloomfield, MO. Photo credits: USACE; http://statehistoricalsocietyofmissouri.org/; Karen Kyle; USFWS http://digitalmedia.fws.gov/cdm/; Cary Aloia This publication printed on recycled paper by ii Contents EXECUTIVE SUMMARY .................................................................................... -

Missouri River Valley in North Eastern Nebraska
Water-Supply Paper No. 2 15 f A, Economic Geology, 96 n I B, Descriptive Geology, 118 owm] M, General Hydrographic Investigations, 25 [ 0, Underground Waters, 71 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR GEOLOGY AND WATER RESOURCES OF A PORTION OF THE MISSOURI RIVER VALLEY IN NORTH EASTERN NEBRASKA BY GK E. WASHINGTON GOVERNMENT PRINTING OFFICE 1908 CONTENTS. Page. Introduction___________________ Topography ___________________ Descriptive geology _____ Stratigraphy ____________ Structure__________________ Description of the rocks____ Cretaceous system ___ Dakota sandstone __ Benton group______ 10 Graueros shale_ 11 Greenhorn limestone 11 Carlile shale_ 12 Niobrara formation 13 Pierre shale_______ 15 Tertiary system________ 18 Arikaree formation- 18 Pliocene deposits_ 19 Quaternary system_____ 20 Glacial drift_______ 20 Loess ________. 20 Alluvium______. 21 Dune sand_________ 22 Economic geology______________ 22 Mineral resources__________ 22 Brick clay ____________ 22 Sand and gravel_______ 22 Building stone_________ 22 Cement rock________ 23 Coal ____________. 2-i Peat __________________ 2-1 Volcanic ash___________ 24 Water resources- __________ 25 General statement _____ 25 Surface waters ________ 25 Streams___________ 25 Springs _______. 20 Underground waters _ 27 Shallow wells _____ 27 Artesian wells_____ 28 Artesian conditions ___________________ 28. Chemical composition of artesian water- 28 Pressure _______________ _ 29 Temperature of artesian water________ 30 Construction and care of -

Boston Borough Strategic Flood Risk Assessment
Water Boston Borough Council October 2010 Strategic Flood Risk Assessment Water Boston Borough Council October 2010 Prepared by: ................................ Checked by: .............................. Roy Lobley Richard Ramsden Associate Director Senior Engineer Approved by: ........................... Andy Yarde Regional Director Strategic Flood Risk Assessment Rev No Comments Checked by Approved Date by 1 Final to client RR AY April 2011 5th Floor, 2 City Walk, Leeds, LS11 9AR Telephone: 0113 391 6800 Website: http://www.aecom.com Job No 60034187 Reference RE01 Date Created October 2010 This document is confidential and the copyright of AECOM Limited. Any unauthorised reproduction or usage by any person other than the addressee is strictly prohibited. f:\projects\50016i boston sfra (revision)\reports\boston sfra final march11.docx Table of Contents Executive Summary ........................................................................................................................................................................ 1 1 Introduction ....................................................................................................................................................................... 7 2 Development Planning...................................................................................................................................................... 9 East Midlands Regional Spatial Strategy ........................................................................................................................... -

Earth Sciences: Two-Ice-Lobe Model for Kansan Glaciation James S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Transactions of the Nebraska Academy of Sciences Nebraska Academy of Sciences and Affiliated Societies 1982 Earth Sciences: Two-Ice-Lobe Model For Kansan Glaciation James S. Aber Emporia State University Follow this and additional works at: http://digitalcommons.unl.edu/tnas Aber, James S., "Earth Sciences: Two-Ice-Lobe Model For Kansan Glaciation" (1982). Transactions of the Nebraska Academy of Sciences and Affiliated Societies. 490. http://digitalcommons.unl.edu/tnas/490 This Article is brought to you for free and open access by the Nebraska Academy of Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Transactions of the Nebraska Academy of Sciences and Affiliated Societies by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 1982. Transactions a/the Nebraska Academy a/Sciences, X:25-29. EARTH SCIENCES TWO-ICE-LOBE MODEL FOR KANSAN GLACIATION James S. Aber Geoscience Department Emporia State University Emporia, Kansas 66801 The Kansan glaciation should be representative of Early Pleisto western source area, the so-called Keewatin Center, in the cene glaciations in the Kansas-Nebraska-Iowa-Missouri region. It is region west of Hudson Bay. Frye and Leonard (1952: 11) often assumed the Kansan ice-sheet advanced as a single, broad lobe supported this concept on the basis of distribution of the coming from somewhere in Canada. This simple view contrasts with the known complexities of the younger Wisconsin glaciation, and indeed Sioux Quartzite, a common erratic derived mainly from east there is much evidence that the Kansan glaciation was equally complex. -

Sauger Population Ecology in Three Missouri River Mainstem Reservoirs Brian D
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 2006 Sauger Population Ecology in Three Missouri River Mainstem Reservoirs Brian D. S. Graeb South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Natural Resources and Conservation Commons Recommended Citation Graeb, Brian D. S., "Sauger Population Ecology in Three Missouri River Mainstem Reservoirs" (2006). Electronic Theses and Dissertations. 438. https://openprairie.sdstate.edu/etd/438 This Dissertation - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. Sauger Population Ecology in Three Missouri River Mainstem Reservoirs By Brian D. S. Graeb A dissertation submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy Major in Biological Sciences South Dakota State University 2006 11 Sauger Population Ecology in Three Missouri River Mainstem Reservoirs This dissertation is approved as a credible and independent investigation by a candidate for the Doctor of Philosophy degree and is acceptable for meeting the dissertation requirements for this degree. Acceptance of this dissertation does not imply that the conclusions reached by the candidate are necessarily the conclusions of the major department. David W. Willis Date Dissertation Advisor Charles G. Scalet Date Head, Department of Wildlife and Fisheries Sciences iii Acknowledgements I would like to especially thank my wife, Stephanie for her support and guidance in all aspects of my life during my long tenure as a graduate student, and my advisor, Dr. -

Ramsar COP8 DOC. 6 Report of the Secretary General Pursuant To
"Wetlands: water, life, and culture" 8th Meeting of the Conference of the Contracting Parties to the Convention on Wetlands (Ramsar, Iran, 1971) Valencia, Spain, 18-26 November 2002 Ramsar COP8 DOC. 6 Report of the Secretary General pursuant to Article 8.2 (b), (c), and (d) concerning the List of Wetlands of International Importance 1. Article 8.2 of the Convention states that: “The continuing bureau duties [the Ramsar Bureau, or convention secretariat] shall be, inter alia : … b) to maintain the List of Wetlands of International Importance and to be informed by the Contracting Parties of any additions, extensions, deletions or restrictions concerning wetlands included in the List provided in accordance with paragraph 5 of Article 21; c) to be informed by the Contracting Parties of any changes in the ecological character of wetlands included in the List provided in accordance with paragraph 2 of Article 32; d) to forward notification of any alterations to the List, or changes in character of wetlands included therein, to all Contracting Parties and to arrange for these matters to be discussed at the next Conference; e) to make known to the Contracting Party concerned, the recommendations of the Conferences in respect of such alterations to the List or of changes in the character of wetlands included therein.” 2. The present report of the Secretary General conveys to the 8th Meeting of the Conference of the Parties the information requested under Article 8 concerning the List of Wetlands of International Importance since the closure of -

Missouri River
Missouri River Planning: Recognizing and Incorporating Sediment Management Committee on Missouri River Recovery and Associated Sediment Management Issues; National Research Council ISBN: 0-309-16204-1, 182 pages, 6 x 9, (2010) This free PDF was downloaded from: http://www.nap.edu/catalog/13019.html Visit the National Academies Press online, the authoritative source for all books from the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council: • Download hundreds of free books in PDF • Read thousands of books online, free • Sign up to be notified when new books are published • Purchase printed books • Purchase PDFs • Explore with our innovative research tools Thank you for downloading this free PDF. If you have comments, questions or just want more information about the books published by the National Academies Press, you may contact our customer service department toll-free at 888-624-8373, visit us online, or send an email to [email protected]. This free book plus thousands more books are available at http://www.nap.edu. Copyright © National Academy of Sciences. Permission is granted for this material to be shared for noncommercial, educational purposes, provided that this notice appears on the reproduced materials, the Web address of the online, full authoritative version is retained, and copies are not altered. To disseminate otherwise or to republish requires written permission from the National Academies Press. Missouri River Planning: Recognizing and Incorporating Sediment Management http://www.nap.edu/catalog/13019.html PREPUBLICATION COPY Missouri River Planning: Recognizing and Incorporating Sediment Management Committee on Missouri River Recovery and Associated Sediment Management Issues Water Science and Technology Board Division of Earth and Life Studies THE NATIONAL ACADEMIES PRESS Washington, D.C. -

Fur Trade on the Missouri
Missouri River Missouri National Recreational River Territory. The second expedition was no more successful, ending at a Ponca village where trader Trade, Tribes, and Transition Antoine Lecuyer took “not less than two wives… and wasted a great deal of the goods of the on the Missouri company.” The company’s third expedition under James MacKay and John Evans successfully The fur trade, America’s first fashion-driven reached the Mandan villages eight years before industry, stimulated Euro-American exploration of Lewis & Clark’s better-known American the Missouri River Valley expedition. several decades before Lewis & Clark. Beaver One of the earliest trappers and traders in the was most important, the Yankton area was Pierre Dorion, Sr., who married dense undercoat being used into the Yankton tribe around 1785 and was a short- to make felt hats. The term member of the Lewis and Clark Expedition in entire world used furs, from 1804. He served as liaison and translator for the wolf to muskrat, for warm clothing in this era council near Calumet Bluff. before synthetic insulating materials. In these early days, independent traders and small Trade was nothing new to the Plains Tribes; the parties were at risk of being relieved of their goods various cultures that occupied this land at different by tribes that chose raiding over trading. Later, times had exchanged produce, dried meat, tools, and large companies built impressive posts that not only weapons for thousands of years. By the mid 1700s, offered protection, but projected an image of the Ihanktonwan Nakota (Yankton Sioux) hosted a permanence that led to long-term trade partnerships. -

Field Guide to the 2008 Farm Bill for Fish and Wildlife Conservation
Field Guide to the 2008 Farm Bill for Fish and Wildlife Conservation A Publication of the U.S. NABCI Committee and the Intermountain West Joint Venture May 2009 Acknowledgements Author: Randall Gray Randall worked for 31 years for the USDA NRCS, where he helped develop, deliver, and evaluate Farm Bill conservation programs. Before retiring, his final position was the National Wildlife Biologist. He is presently the Farm Bill Coordinator for the Intermountain West Joint Venture. Financial Contributions: Association of Fish and Wildlife Agencies Intermountain West Joint Venture The Nature Conservancy, Migratory Bird Program Gray Wildlife Consulting U.S. Fish and Wildlife Service The following contributed to or reviewed this guide: Roxanne Bogart, U.S. Fish and Wildlife Service Wes Burger, Mississippi State University Barth Crouch, Playa Lakes Joint Venture Leslie Deavers, USDA Natural Resources Conservation Service Terrell Erickson, USDA Natural Resources Conservation Service Deborah Hahn, Association of Fish and Wildlife Agencies Jon Haufler, Ecosystem Management Research Institute Steve Hilburger, United States Geological Survey Luke Miller, Ohio Department of Natural Resources Sal Palazzolo, Arizona Game and Fish Department Matthew Ponish, USDA Farm Services Agency Charlie Rewa, USDA, Natural Resources Conservation Service Steve Riley, Nebraska Game and Parks Commission Dave Smith, Intermountain West Joint Venture Reggie Thackston, Georgia Department of Natural Resources Bill White, Missouri Department of Conservation USDA Natural Resources -

Drainage Patterns and Glaciations in Eastern Nebraska William J
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in the Earth and Atmospheric Sciences Earth and Atmospheric Sciences, Department of 1985 Drainage Patterns and Glaciations in Eastern Nebraska William J. Wayne University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/geosciencefacpub Part of the Earth Sciences Commons Wayne, William J., "Drainage Patterns and Glaciations in Eastern Nebraska" (1985). Papers in the Earth and Atmospheric Sciences. 562. https://digitalcommons.unl.edu/geosciencefacpub/562 This Article is brought to you for free and open access by the Earth and Atmospheric Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in the Earth and Atmospheric Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. ... Institute for Tertiary-Quaternary Studies-TER-QUA Symposium Series, 1: 111-117. (Lq Rs) Drainage Patterns and Glaciations in Eastern Nebraska William J. Wayne Department of Geology University of Nebraska-Lincoln Lincoln, Nebraska 68588-0340 Most of the stream courses of eastern Nebraska came into exis fluviatile sediments across Nebraska and the adjacent states tence during the recession of an ice margin. Their patterns include changed many times during that period, but the alluvial many drainage anomalies that suggest both the manner and the times of surface continued to slope eastward, and the drainage lines ice disappearance. Rivers coming from the west flow down the regional slope of the High Plains. Along and east of the "Kansan" glacial border followed that slope. 0 most streams are subparallel, trend S300 _40 E, and probablY mark At the time the first of the Early Pleistocene continental pauses of an actively retreating ice margin.