Beyond Fishing in the Newfoundland and Labrador Marine Shelf Ecosystem: the Roles of Climate and Environment in Affecting Change
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Hitch-Hiker Is Intended to Provide Information Which Beginning Adult Readers Can Read and Understand
CONTENTS: Foreword Acknowledgements Chapter 1: The Southwestern Corner Chapter 2: The Great Northern Peninsula Chapter 3: Labrador Chapter 4: Deer Lake to Bishop's Falls Chapter 5: Botwood to Twillingate Chapter 6: Glenwood to Gambo Chapter 7: Glovertown to Bonavista Chapter 8: The South Coast Chapter 9: Goobies to Cape St. Mary's to Whitbourne Chapter 10: Trinity-Conception Chapter 11: St. John's and the Eastern Avalon FOREWORD This book was written to give students a closer look at Newfoundland and Labrador. Learning about our own part of the earth can help us get a better understanding of the world at large. Much of the information now available about our province is aimed at young readers and people with at least a high school education. The Hitch-Hiker is intended to provide information which beginning adult readers can read and understand. This work has a special feature we hope readers will appreciate and enjoy. Many of the places written about in this book are seen through the eyes of an adult learner and other fictional characters. These characters were created to help add a touch of reality to the printed page. We hope the characters and the things they learn and talk about also give the reader a better understanding of our province. Above all, we hope this book challenges your curiosity and encourages you to search for more information about our land. Don McDonald Director of Programs and Services Newfoundland and Labrador Literacy Development Council ACKNOWLEDGMENTS I wish to thank the many people who so kindly and eagerly helped me during the production of this book. -

Rental Housing Portfolio March 2021.Xlsx
Rental Housing Portfolio Profile by Region - AVALON - March 31, 2021 NL Affordable Housing Partner Rent Federal Community Community Housing Approved Units Managed Co-op Supplement Portfolio Total Total Housing Private Sector Non Profit Adams Cove 1 1 Arnold's Cove 29 10 39 Avondale 3 3 Bareneed 1 1 Bay Bulls 1 1 10 12 Bay Roberts 4 15 19 Bay de Verde 1 1 Bell Island 90 10 16 116 Branch 1 1 Brigus 5 5 Brownsdale 1 1 Bryants Cove 1 1 Butlerville 8 8 Carbonear 26 4 31 10 28 99 Chapel Cove 1 1 Clarke's Beach 14 24 38 Colinet 2 2 Colliers 3 3 Come by Chance 3 3 Conception Bay South 36 8 14 3 16 77 Conception Harbour 8 8 Cupids 8 8 Cupids Crossing 1 1 Dildo 1 1 Dunville 11 1 12 Ferryland 6 6 Fox Harbour 1 1 Freshwater, P. Bay 8 8 Gaskiers 2 2 Rental Housing Portfolio Profile by Region - AVALON - March 31, 2021 NL Affordable Housing Partner Rent Federal Community Community Housing Approved Units Managed Co-op Supplement Portfolio Total Total Housing Goobies 2 2 Goulds 8 4 12 Green's Harbour 2 2 Hant's Harbour 0 Harbour Grace 14 2 6 22 Harbour Main 1 1 Heart's Content 2 2 Heart's Delight 3 12 15 Heart's Desire 2 2 Holyrood 13 38 51 Islingston 2 2 Jerseyside 4 4 Kelligrews 24 24 Kilbride 1 24 25 Lower Island Cove 1 1 Makinsons 2 1 3 Marysvale 4 4 Mount Carmel-Mitchell's Brook 2 2 Mount Pearl 208 52 18 10 24 28 220 560 New Harbour 1 10 11 New Perlican 0 Norman's Cove-Long Cove 5 12 17 North River 4 1 5 O'Donnels 2 2 Ochre Pit Cove 1 1 Old Perlican 1 8 9 Paradise 4 14 4 22 Placentia 28 2 6 40 76 Point Lance 0 Port de Grave 0 Rental Housing Portfolio Profile by Region - AVALON - March 31, 2021 NL Affordable Housing Partner Rent Federal Community Community Housing Approved Units Managed Co-op Supplement Portfolio Total Total Housing Portugal Cove/ St. -
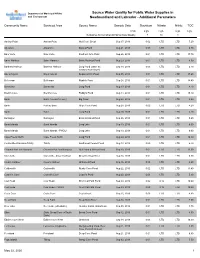
Source Water Quality for Public Water Supplies in Newfoundland And
Department of Municipal Affairs Source Water Quality for Public Water Supplies in and Environment Newfoundland and Labrador - Additional Parameters Community Name Serviced Area Source Name Sample Date Strontium Nitrate Nitrite TOC Units mg/L mg/L mg/L mg/L Guidelines for Canadian Drinking Water Quality 7 10 1 Anchor Point Anchor Point Well Cove Brook Sep 17, 2019 0.02 LTD LTD 7.20 Aquaforte Aquaforte Davies Pond Aug 21, 2019 0.00 LTD LTD 6.30 Baie Verte Baie Verte Southern Arm Pond Sep 26, 2019 0.01 LTD LTD 17.70 Baine Harbour Baine Harbour Baine Harbour Pond Aug 29, 2019 0.01 LTD LTD 9.50 Bartletts Harbour Bartletts Harbour Long Pond (same as Sep 18, 2019 0.03 LTD LTD 6.70 Castors River North) Bay L'Argent Bay L'Argent Sugarloaf Hill Pond Sep 05, 2019 0.01 LTD LTD 17.60 Belleoram Belleoram Rabbits Pond Sep 24, 2019 0.01 LTD LTD 14.40 Bonavista Bonavista Long Pond Aug 13, 2019 0.01 LTD LTD 4.10 Brent's Cove Brent's Cove Paddy's Pond Aug 14, 2019 0.01 LTD LTD 15.10 Burin Burin (+Lewin's Cove) Big Pond Aug 28, 2019 0.01 LTD LTD 4.90 Burin Port au Bras Gripe Cove Pond Aug 28, 2019 0.02 LTD LTD 4.20 Burin Burin Long Pond Aug 28, 2019 0.01 LTD LTD 4.10 Burlington Burlington Eastern Island Pond Sep 26, 2019 0.01 LTD LTD 9.60 Burnt Islands Burnt Islands Long Lake Sep 10, 2019 0.01 LTD LTD 6.00 Burnt Islands Burnt Islands - PWDU Long Lake Sep 10, 2019 0.01 LTD LTD 6.00 Cape Freels North Cape Freels North Long Pond Aug 20, 2019 0.01 LTD LTD 10.30 Centreville-Wareham-Trinity Trinity Southwest Feeder Pond Aug 13, 2019 0.00 LTD LTD 6.70 Channel-Port -

Community Files in the Centre for Newfoundland Studies
Community Files in the Centre for Newfoundland Studies A | B | C | D | E | F | G | H | I | J | K | L | M | N | 0 | P | Q-R | S | T | U-V | W | X-Y-Z A Abraham's Cove Adams Cove, Conception Bay Adeytown, Trinity Bay Admiral's Beach Admiral's Cove see Port Kirwan Aguathuna Alexander Bay Allan’s Island Amherst Cove Anchor Point Anderson’s Cove Angel's Cove Antelope Tickle, Labrador Appleton Aquaforte Argentia Arnold's Cove Aspen, Random Island Aspen Cove, Notre Dame Bay Aspey Brook, Random Island Atlantic Provinces Avalon Peninsula Avalon Wilderness Reserve see Wilderness Areas - Avalon Wilderness Reserve Avondale B (top) Baccalieu see V.F. Wilderness Areas - Baccalieu Island Bacon Cove Badger Badger's Quay Baie Verte Baie Verte Peninsula Baine Harbour Bar Haven Barachois Brook Bareneed Barr'd Harbour, Northern Peninsula Barr'd Islands Barrow Harbour Bartlett's Harbour Barton, Trinity Bay Battle Harbour Bauline Bauline East (Southern Shore) Bay Bulls Bay d'Espoir Bay de Verde Bay de Verde Peninsula Bay du Nord see V.F. Wilderness Areas Bay L'Argent Bay of Exploits Bay of Islands Bay Roberts Bay St. George Bayside see Twillingate Baytona The Beaches Beachside Beau Bois Beaumont, Long Island Beaumont Hamel, France Beaver Cove, Gander Bay Beckford, St. Mary's Bay Beer Cove, Great Northern Peninsula Bell Island (to end of 1989) (1990-1995) (1996-1999) (2000-2009) (2010- ) Bellburn's Belle Isle Belleoram Bellevue Benoit's Cove Benoit’s Siding Benton Bett’s Cove, Notre Dame Bay Bide Arm Big Barasway (Cape Shore) Big Barasway (near Burgeo) see -

THE OCCURRENCE of GREENLAND and EUROPEAN BIRDS in NEWFOUNDLAND by L•Slm M
184] J. O.L. Roberts Bird-BandingJuly, 1971 CALL EPSIL (S,T,M,P) 1 E = E +FLOAT(N)*FLOAT(NB)*S**NC*X +FLOAT(N)*S**NB* XA*2. +S**N*P RETURN END C SAMPLE DATA 1 6 1810 5 2 0 0 864511 1 ß435 35 THE OCCURRENCE OF GREENLAND AND EUROPEAN BIRDS IN NEWFOUNDLAND By L•sLm M. TUCK* Newfoundland is a large island of 42,734 square miles situated in the North Atlantic Ocean between 46 ø and 52 ø north latitude. The Avalon Peninsula, its most southeasterly portion, is in the same latitude as the Bay of Biscay, France. Newfoundlandis some1,650 miles from the coast of Ireland, the nearest land in Europe. It is 850 miles from Cape Farewell, the most southerly point in Greenland. The physiographyof Newfoundland is quite similar to that of the adjacent Maritime Provinces. The coast is indented with bays and inlets and the island itself is a plateau which, sloping in a north- westerly direction, reacheselevations up to 1,500 feet in the almost mountainoushighlands along the west coast. A large part of the island's terrain is bleak and the soil is relatively shallow. In general, commercial forests are confined to the river valleys. Becauseit is on the eastern side of the North American continent, Newfoundland is influenced by continental air massesand exper- iences a wide range of summer and winter temperatures. More- over, as the island is virtually encircledby the cold waters of the Labrador Current, the sea moderatessummer and winter tempera- tures. Sea-icefrom the arctic regionsreaches Newfoundland in January (Figure 1) and in a normal year may surround the entire island except the south coast. -
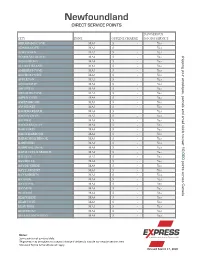
Newfoundland DIRECT SERVICE POINTS
Newfoundland DIRECT SERVICE POINTS DANGEROUS CITY ZONE OFFLINE CHARGE GOODS SERVICE ABRAHAMS COVE MA4 $ - Yes ADAMS COVE MA3 $ - Yes ADEYTOWN MA3 $ - Yes ADMIRALS BEACH MA4 $ - Yes shipping your envelopes, parcels and small skids to over AGUATHUNA MA4 $ - Yes ALLANS ISLAND MA3 $ - Yes AMHERST COVE MA3 $ - Yes ANCHOR POINT MA4 $ - Yes APPLETON MA3 $ - Yes AQUAFORTE MA4 $ - Yes ARGENTIA MA4 $ - Yes ARNOLDS COVE MA3 $ - Yes ASPEN COVE MA4 $ - Yes ASPEY BROOK MA3 $ - Yes AVONDALE MA3 $ - Yes BACK HARBOUR MA4 $ - Yes BACON COVE MA3 $ - Yes BADGER MA4 $ - Yes BADGERS QUAY MA4 $ - Yes BAIE VERTE MA4 $ - Yes BAINE HARBOUR MA4 $ - Yes BARACHOIS BROOK MA4 $ - Yes BARENEED MA3 $ - Yes BARRD ISLANDS MA4 $ - Yes 10,000 BARTLETTS HARBOUR MA4 $ - Yes BAULINE MA3 $ - Yes BAY BULLS MA4 $ - Yes points across Canada BAY DE VERDE MA4 $ - Yes BAY L'ARGENT MA4 $ - Yes BAY ROBERTS MA3 $ - Yes BAYSIDE MA4 $ - Yes BAYTONA MA4 $ - Yes BAYVIEW MA4 $ - Yes BEACHES MA4 $ - Yes BEACHSIDE MA4 $ - Yes BEAR COVE MA4 $ - Yes BEAU BOIS MA3 $ - Yes BEAUMONT MA4 $ - Yes BELL ISLAND FRONT MA4 $ - Yes Notes: Some points not serviced daily. Shipments may be subject to a beyond charge if delivery is outside our regular service area. Standard Terms & Conditions will apply. Revised March 17, 2020 Newfoundland DIRECT SERVICE POINTS DANGEROUS CITY ZONE OFFLINE CHARGE GOODS SERVICE BELL ISLAND MA4 $ - Yes BELLBURNS MA4 $ - Yes BELLEORAM MA4 $ - Yes BELLEVUE MA4 $ - Yes shipping your envelopes, parcels and small skids to over BELLMANS COVE MA4 $ - Yes BENOITS COVE MA4 $ - Yes BENTON -

Private Sector Non Profit Adams Cove 1 1 Arnold's Cove 9 10 20 39 Avondale 3 4 7 Bareneed 1 1 Bay Bulls 1 2 10 13 Bay Roberts 2
Rental Housing Portfolio Profile by Region - AVALON - March 31, 2019 Affordable Housing Approved Partner NL Housing Rent Federal Community Community Units Managed Co-op Total Supplement Portfolio Total Housing Private Sector Non Profit Adams Cove 1 1 Arnold's Cove 9 10 20 39 Avondale 3 4 7 Bareneed 1 1 Bay Bulls 1 2 10 13 Bay Roberts 2 15 17 Bay de Verde 1 1 Bell Island 88 10 16 114 Branch 1 1 Brigus 5 5 Brownsdale 1 1 Bryants Cove 1 1 Butlerville 8 8 Carbonear 26 1 27 10 28 92 Chapel Cove 1 1 Clarke's Beach 14 24 38 Colinet 2 2 Colliers 3 3 Come by Chance 3 3 Conception Bay South 36 10 20 3 16 85 Conception Harbour 8 8 Cupids 8 8 Cupids Crossing 1 1 Dildo 1 1 Dunville 11 11 Ferryland 6 6 Fox Harbour 1 1 Freshwater, P. Bay 8 8 Gaskiers 2 2 Goobies 2 2 Rental Housing Portfolio Profile by Region - AVALON - March 31, 2019 Affordable Housing Approved Partner NL Housing Rent Federal Community Community Units Managed Co-op Total Supplement Portfolio Total Housing Goulds 8 3 11 Green's Harbour 2 2 Harbour Grace 14 1 16 31 Harbour Main 1 1 Heart's Content 2 2 Heart's Delight 3 12 15 Heart's Desire 2 2 Holyrood 13 1 53 67 Islingston 2 2 Jerseyside 4 4 Kelligrews 40 40 Kilbride 1 24 25 Lower Island Cove 1 1 Makinsons 2 1 3 Marysvale 4 4 Mount Carmel-Mitchell's Brook 2 2 Mount Pearl 208 45 24 2 24 192 220 715 New Harbour 1 17 18 New Perlican 0 Norman's Cove-Long Cove 5 12 17 North River 4 1 5 O'Donnels 2 2 Ochre Pit Cove 1 1 Old Perlican 1 8 9 Paradise 3 60 4 67 Placentia 28 2 20 40 90 Point Lance 0 Port de Grave 0 Portugal Cove/ St. -

Office Allowances - Office Accommodations 01-Apr-15 to 31-Mar-16
House of Assembly Newfoundland and Labrador Member Accountability and Disclosure Report Office Allowances - Office Accommodations 01-Apr-15 to 31-Mar-16 Bennett, Derek, MHA Page: 1 of 1 Summary of Transactions Processed to Date for Fiscal 2015/16 Transactions Processed as of: 31-Mar-16 Expenditures Processed to Date (Net of HST): $0.00 Date Source Document # Vendor Name Expenditure Details Amount Period Activity: 0.00 Opening Balance: 0.00 Ending Balance: 0.00 ---- End of Report ---- House of Assembly Newfoundland and Labrador Member Accountability and Disclosure Report Office Allowances - Rental of Short-term Accommodations 01-Apr-15 to 31-Mar-16 Bennett, Derek, MHA Page: 1 of 1 Summary of Transactions Processed to Date for Fiscal 2015/16 Transactions Processed as of: 31-Mar-16 Expenditures Processed to Date (Net of HST): $0.00 Date Source Document # Vendor Name Expenditure Details Amount Period Activity: 0.00 Opening Balance: 0.00 Ending Balance: 0.00 ---- End of Report ---- House of Assembly Newfoundland and Labrador Member Accountability and Disclosure Report Office Allowances - Office Start-up Costs 01-Apr-15 to 31-Mar-16 Bennett, Derek, MHA Page: 1 of 1 Summary of Transactions Processed to Date for Fiscal 2015/16 Expenditure Limit (Net of HST): $885.00 Transactions Processed as of: 31-Mar-16 Expenditures Processed to Date (Net of HST): $290.20 Funds Available (Net of HST): $594.80 Percent of Funds Expended to Date: 32.8% Date Source Document # Vendor Name Expenditure Details Amount 07-Jan-16 40380243 CORPORATE EXPRESS Staples Push Pins, Item # STP14429. 0.74 CANADA, INC 08-Jan-16 J236779 GRAND & TOY LTD Quartet Cork Bulletin Board, Item # 2303. -

Private Sector Non Profit Adams Cove 1 1 Arnold's Cove 29 10 39
Rental Housing Portfolio Profile by Region - AVALON - March 31, 2020 NL Affordable Housing Partner Rent Federal Community Community Housing Approved Units Managed Co-op Supplement Portfolio Total Total Housing Private Sector Non Profit Adams Cove 1 1 Arnold's Cove 29 10 39 Avondale 3 4 7 Bareneed 1 1 Bay Bulls 1 1 10 12 Bay Roberts 0 1 15 16 Bay de Verde 1 1 Bell Island 89 10 16 115 Branch 1 1 Brigus 5 5 Brownsdale 1 1 Bryants Cove 1 1 Butlerville 8 8 Carbonear 26 4 31 10 28 99 Chapel Cove 1 1 Clarke's Beach 14 24 38 Colinet 2 2 Colliers 3 3 Come by Chance 3 3 Conception Bay South 36 10 14 3 16 79 Conception Harbour 8 8 Cupids 8 8 Cupids Crossing 1 1 Dildo 1 1 Dunville 11 11 Ferryland 6 6 Fox Harbour 1 1 Freshwater, P. Bay 8 8 Gaskiers 2 2 Goobies 2 2 Goulds 8 4 12 Green's Harbour 2 2 Harbour Grace 14 1 16 31 Rental Housing Portfolio Profile by Region - AVALON - March 31, 2020 NL Affordable Housing Partner Rent Federal Community Community Housing Approved Units Managed Co-op Supplement Portfolio Total Total Housing Harbour Main 1 1 Heart's Content 2 2 Heart's Delight 3 14 17 Heart's Desire 2 2 Holyrood 13 38 51 Islingston 0 Jerseyside 4 4 Kelligrews 30 30 Kilbride 1 1 Lower Island Cove 1 1 Makinsons 2 1 3 Marysvale 4 4 Mount Carmel-Mitchell's Brook 2 2 Mount Pearl 208 55 24 10 24 52 220 593 New Harbour 1 7 8 New Perlican 0 Norman's Cove-Long Cove 5 12 17 North River 4 1 5 O'Donnels 2 2 Ochre Pit Cove 1 1 Old Perlican 1 8 9 Paradise 3 44 4 51 Placentia 28 2 6 40 76 Point Lance 0 Port de Grave 0 Portugal Cove/ St. -

Timelines of Newfoundland and Labrador
Timelines of Newfoundland and Labrador Newfoundland and Labrador Adult Basic Education Social History Series A Joint Project of The Writers' Alliance of Newfoundland and Labrador and Cabot College Literacy Office In This Series... Book 1 - Timelines of Newfoundland and Labrador Book 2 - Facing the New Economy Book 3 - Learning About the Past Book 4 - Desperate Measures The Great Depression in Newfoundland and Labrador Book 5 - Health and Hard Time Book 6 - Multicultural History Book 7 - Surviving in Rural Newfoundland Book 8 - The Struggle for Work in the Great Depression Book 9 - How Long do I Have to Wait? Book 10 - William Pender The Story of a Cooper Book 1: Timelines of Newfoundland and Labrador © Copyright 1996-Writers' Alliance of Newfoundland and Labrador All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without the written permission of the Writers' Alliance of Newfoundland and Labrador. Box 2681 St. John's, Newfoundland A1C 5M5 Lay-out and Design: Walbourne Design Associates Ltd. Thanks to the National Literacy Secretariat and the Canada/Newfoundland Cooperation Agreement on Cultural Industries for funding this project. Canadian Cataloguing in Publication Data Main entry under title: Newfoundland adult basic education social history series. Partial contents: Bk. 1. Timelines of Newfoundland and Labrador. ISBN 1-896858-00-7 (bk. 1) 1. Readers for new literates. 2. Readers (Adult). 3. Newfoundland-History. 4. Labrador-History. 5. Writers' Alliance of Newfoundland and Labrador. PE1 126.A4N48 1996 428.6'2 C96-950124-2 Printed in Newfoundland and Labrador by Robinson Blackmore Printing Ltd, St. -

Tap Water Quality for Public Water Supplies in Newfoundland and Labrador and Environment Physical Parameters and Major Ions
Department of Municipal Affairs Tap Water Quality for Public Water Supplies in Newfoundland and Labrador and Environment Physical Parameters and Major Ions Serviced Area(s) Source Name Sample Date Alkalinity Colour Conductivity Hardness pH TDS TSS Turbidity Boron Bromide Calcium Chloride Fluoride Potassium Sodium Sulphate Units mg/L TCU µS/cm mg/L mg/L mg/L NTU mg/L mg/L mg/L mg/L mg/L mg/L mg/L mg/L 15 6.5 - 8.5 500 1.0 5.0 250 1.5 200 500 Guidelines for Canadian Drinking Water Quality Aesthetic (A) or Contaminant (C) Parameter A A A C C A C A A Anchor Point Anchor Point Well Cove Brook Sep 17, 2019 106.00 10 243.0 120.00 8.28 158 0.60 LTD LTD 25.00 13 LTD LTD 7 1 Appleton Appleton (+Glenwood) Gander Lake (The Outflow) Aug 12, 2019 LTD 26 33.0 2.00 5.16 21 0.60 LTD LTD 1.00 7 LTD LTD LTD 1 Aquaforte Aquaforte Davies Pond Aug 21, 2019 12.00 14 107.0 LTD 7.79 70 0.40 LTD LTD LTD 24 LTD LTD 14 2 Arnold's Cove Arnold's Cove Steve's Pond (2 Intakes) Aug 21, 2019 12.00 10 61.0 5.00 7.21 40 0.60 LTD LTD 2.00 13 LTD LTD 9 1 Avondale Avondale Lee's Pond Sep 05, 2019 14.00 2 88.0 12.00 8.25 57 0.40 LTD LTD 5.00 15 LTD LTD 11 1 Baie Verte Baie Verte Southern Arm Pond Sep 26, 2019 LTD 19 34.0 5.00 5.05 22 0.20 LTD LTD 2.00 7 LTD LTD 2 1 Baine Harbour Baine Harbour Baine Harbour Pond Aug 29, 2019 8.00 83 38.0 5.00 7.33 25 0.40 LTD LTD 2.00 4 LTD LTD 4 2 Bartletts Harbour Bartletts Harbour Long Pond (same as Castors Sep 18, 2019 96.00 23 265.0 112.00 8.42 172 0.50 LTD LTD 25.00 22 LTD LTD 12 3 River North) Bauline Bauline #1 Brook Path Well Aug 15, -

Acknowledgements
ACKNOWLEDGEMENTS Acknowledgements The Department of Education acknowledges the collaborative efforts of many individuals who contributed their time, efforts, and suggestions during various stages of the developement of this curriculum guide. Appreciation is expressed to all of them. Joan Casey, Program Development Specialist, Division of Program Development, Department of Education, coordinator of the pilot process and editor of the document. Recognition is given to Suzelle Lavallée, Program Development Specialist, Division of Program Development, Department of Education, for contribution to editing the outcomes. Tanya Smith, Teacher, Riverwood Academy, Wing’s Point, Gander Bay, writer of the pilot draft of the curriculum guide and member of the pilot inservice team. Chris Vincent, Teacher, Lewisporte Academy, Lewisporte, author of the pilot draft of the curriculum guide, member of the pilot inservice team, and editor of the document. Special appreciation is extended to the teachers who piloted the program. Their valuable comment and suggestions are reflected in the curriculum guide: Andrew Battcock. St. Michael’s School, Goose Bay; John Boland, J. J. Curling Elementary, Corner Brook; Krista Mercer, St. Francis Elementary, Parson’s Pond; Chris Osmond, Newville Elementary, Herring Neck and Summerford Elementary, Summerford; Cliff Powell, St. Anthony Elementary, St. Anthony; Deborah Sheppard, B.L. Morrison School, Postville; and Howard Stevens, St. Genevieve Elementary, Plum Point. PHYSICAL EDUCATION CURRICULUM GUIDE - PRIMARY AND ELEMENTARY i ACKNOWLEDGEMENTS Appreciation is extended to School District personnel who facilitated the pilot process: Darryl Feener, Program Specialist, Labrador; Betty King, Program Specialist, Corner Brook/Deer Lake/St. Barbe; Renee Sherstrobetoff, Program Specialist, Corner Brook/Deer Lake/St. Barbe; Harrison Smith, Program Specialist, Northern Peninsula/Labrador South; and Diane Vey-Morawski, Program Specialist, Lewisporte/Gander.