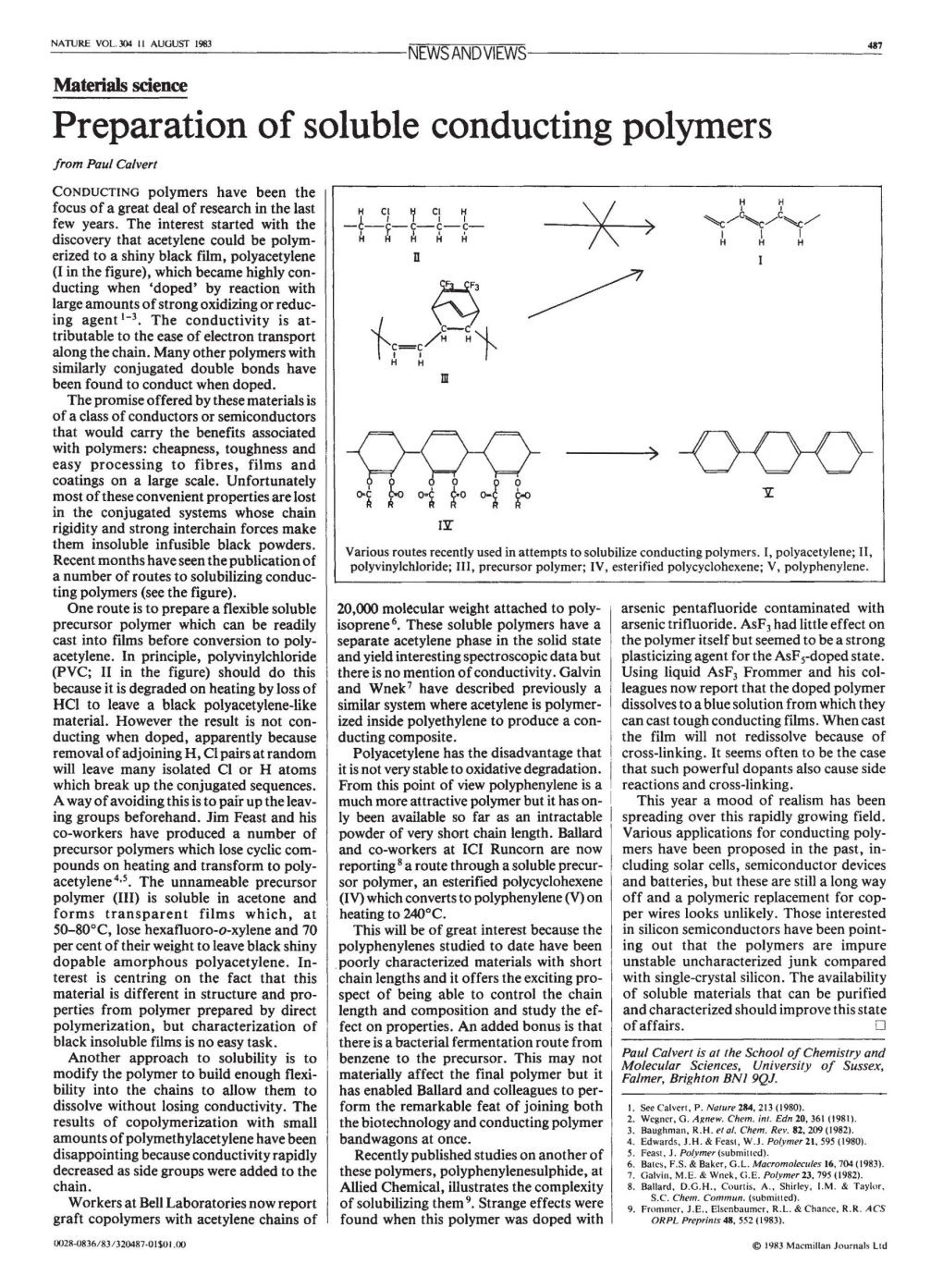
Preparation of Soluble Conducting Polymers from Paul Calvert CONDUCTING Polymers Have Been the Focus of a Great Deal of Research in the Last H C1 ~ C1 ~ Few Years
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Safety Manual
LABORATORY SAFETY MANUAL Environment, Health & Safety 1120 Estes Drive Extension CB# 1650 TABLE OF CONTENTS Section or Chapter Page Introduction i Emergency Telephone Numbers ii EHS – Scope of Service iii Condensed Laboratory Safety Information for New Research Personnel v Chapter 1 – Laboratory Safety at the University of North Carolina at Chapel Hill 1-1 Chapter 2 – Laboratory Safety Plan 2-1 Chapter 3 – General Safety Principles and Practices 3-1 Chapter 4 – Proper Storage of Chemicals in Laboratories 4-1 Chapter 5 – Protective Clothing and Equipment 5-1 Chapter 6 – Safe Handling of Chemicals 6-1 Chapter 7 – Highly Toxic Chemicals and Select Carcinogens 7-1 Chapter 8 – Reproductive Health 8-1 Chapter 9 – Controlled Substances 9-1 Chapter 10 – Fire Safety 10-1 Chapter 11 – Explosive and Reactive Chemical Hazards 11-1 Chapter 12 – Management of Laboratory Wastes 12-1 Chapter 13 – Safe Handling of Peroxidizable Compounds 13-1 Chapter 14 – Safe Handling of Laboratory Animals 14-1 Chapter 15 – Safe Handling of Biological Hazards 15-1 Chapter 16 – Biological Safety Cabinets 16-1 Chapter 17 – Laboratory Hoods 17-1 Chapter 18 – Safe Use of Nanomaterials 18-1 Revisions to Laboratory Safety Manual REV-1 Laboratory Safety Manual – the University of North Carolina at Chapel Hill INTRODUCTION This manual is a safety reference document for laboratory personnel at the University of North Carolina at Chapel Hill. The University’s Department of Environment, Health & Safety prepared this manual, followed by review and approval from both the University’s Laboratory and Chemical Safety Committee (LCSC) and the University Safety and Security Committee (USSC). -

1 Synthesis and Functionality of Substituted Polyacetylenes Jianzhao Liu, Jacky W
1 1 Synthesis and Functionality of Substituted Polyacetylenes Jianzhao Liu, Jacky W. Y. Lam, and Ben Zhong Tang 1.1 Introduction Polyacetylene (PA) is the archetypal conjugated polymer. The seminal discovery of the metallic conductivity of its doped form has triggered a huge surge of interest in conductive polymers and has spawned an exciting area of research on synthetic metals.1) As a result of rapid advances in the area, we are now on the threshold of a ‘‘plastic-electronics era’’ that previously could only be imagined in science fiction. Structurally, PA is a linear polyene chain [−(HC=CH)n−]. The existence of two hydrogen atoms in its repeat unit offers ample opportunity to decorate the backbone with pendants: replacement of hydrogen in each repeat unit by one or two substituents yields mono- (1) and disubstituted PAs (2), respectively (Scheme 1.1). The pendant and backbone can interact with each other: for example, the former perturbs the electronic conjugation of the latter, while the latter in- fluences the molecular alignment of the former. Proper structural design may tune the backbone–pendant interplay into harmony and synergy, generating new substituted PAs with novel functionalities. While PA is electrically conductive, introduction of such pendant as mesogen, chromophore, photosensitive double bond, or naturally occurring building block may endow it with such new func- tional properties as electro-optic activity, photonic responsiveness, and biologic compatibility. Considerable efforts have been devoted to the synthesis of the substituted PAs and study of their properties [1–9]. Attachment of polar groups to the polyene backbone enables the integration of functional pendants into the PA chain through various functional-group transformations, which was, however, a difficult task at the early stage of PA chemistry. -

Electronic Polymers
Electronic Polymers Insulators Semiconductors Metals Superconductors σ <10-7 10−7< σ <102 σ >102 σ >>1020 σ ranges 10-20 to 1020 Requires doping (oxidation or reduction) for conductivity Electrical Properties Electric conductivity of inorganic (I) and organic (O) compounds, I O measured in S/cm. Triniobium germanide (Nb3Ge) and poly(thiazyl) (SN)n are superconducting materials at very low temperatures near 20 zero kelvin. The conductivities for conducting (C), semi-conducting S Nb3Ge, (SN)n 10 (SC) and insulating (I) compounds are given for 20oC (= 293.16 K = 68oF). Cu = Copper, Hg = mercury, Ge = germanium, Si = silicon, 1015 AgBr = silver bromide, G = glass, S = sulfur, (SiO2)n= quartz, TTF = tetrathiafulvalene, TCNQ = 7,7,8,8 tetracyanoquinodimethane, NBR C 1010 = nitrile rubber (a copolymer from acrylonitrile and butadiene), DNA = deoxyribonucleic acid, PVC = polyvinyl chloride, PE = polyethylene, PTFE = polytetrafluoroethylene. Cu 105 TTF/TCNQ Hg 1 Siemens = 1 Ohm-1 (SN)n 1 Ge 1040 change in material property ! SC dP Si 10-5 AgBr Material Conductivity (S/cm) NBR -10 G 10 -7 DNA Insulators σ < 10 PVC 10-15 Semiconductors 10−7 < σ < 102 I S (SiO ) PE 2 n Metals σ > 102 10-20 PTFE Superconductors σ >> 1020 n = # carriers/cm3 Figure by MIT OCW. σ = nμq μ= mobility (cm2/V•sec) q = charge Types of Charge • Usual carriers: electrons, holes, ions(cations & anions) • New for conducting polymers – solitons, polarons, bipolarons Ji = σijEj where Ji is the current, σij is the conductivity and Ej is the applied field Battery Application – Li-polymer vs Pb. – Weight: 1/10th – volume: 1/3rd – power density: 10x – processable into any shape; dry, no toxic fumes etc. -

Synthetic and Mechanistic Studies of Poly(Vinyl Chloride) and Some Other Chlorinated Polymers XIANLONG GE College of William &A
Synthetic and Mechanistic Studies of Poly(vinyl chloride) and Some Other Chlorinated Polymers XIANLONG GE College of William & Mary, Department of Applied Science, 2003 Field: Polymer Science, Degree: Ph.D. Advisor: William H. Starnes, Jr., Professor of Chemistry Abstract Poly(1,2-dichloroethylene) (PDCE) is an unknown polymer that should be a superb engineering thermoplastic for use in a variety of high-performance applications. This thesis discusses approaches to its synthesis and describes the preparation and some of the properties of a number of new polymers that contain chlorine. Other major topics addressed here are (a) the involvement of an excited cation diradical intermediate in the thermal degradation of poly(vinyl chloride) (PVC) and (b) the mechanism of the thermal stabilization of PVC by “plasticizer thiols”. Unlike vinyl chloride, 1,2-dchloroethylene (DCE) undergoes dimerization under free-radical conditions. Chain transfer to DCE by β-Cl elimination was shown to be the major reason for its nonpolymerization. The dimeric radical rearranges by a 1,2-Cl shift, but apparently to only a very minor extent. During the chlorination of alkyl chlorides with molecular chlorine, a bridged intermediate is involved, and for this reason, vicinal chlorides were found to be the major products. The yields of geminal chlorides increased significantly in the presence of solvents that form complexes with chlorine atoms, but such solvents also decreased the reactivity of the chlorination. Thus the chlorination of PVC in the presence of complexing solvents was not a useful method for the synthesis of PDCE Polyacetylene (PA) was prepared by the methods of both Shirakawa and Luttinger. -

Polyacetylene: Myth and Reality
materials Review Polyacetylene: Myth and Reality Bruce S. Hudson Department of Chemistry, Syracuse University, Syracuse, NY 13244-4100, USA; [email protected]; Tel.: +1-315-443-5805 Received: 31 December 2017; Accepted: 31 January 2018; Published: 6 February 2018 Abstract: Polyacetylene, the simplest and oldest of potentially conducting polymers, has never been made in a form that permits rigorous determination of its structure. Trans polyacetylene in its fully extended form will have a potential energy surface with two equivalent minima. It has been assumed that this results in bond length alternation. It is, rather, very likely that the zero-point energy is above the Peierls barrier. The experimental studies that purport to show bond alternation are reviewed and shown to be compromised by serious experimental inconsistencies or by the presence, for which there is considerable evidence, of finite chain polyenes. In this view, addition of dopants results in conductivity by facilitation of charge transport between finite polyenes. The double minimum potential that necessarily occurs for polyacetylene, if viewed as the result of elongation of finite 1 1 chains, originates from admixture of the 1 Ag ground electronic state with the 2 Ag excited electronic singlet state. This excitation is diradical (two electron) in character. The polyacetylene limit is an 1 equal admixture of these two Ag states making theory intractable for long chains. A method is outlined for preparation of high molecular weight polyacetylene with fully extended chains that are prevented from reacting with neighboring chains. Keywords: polyacetylene; double-minimum potential; Peierls barrier; zero-point level; cross-linking 1. Introduction/Background History Polyacetylene is selected for review because of its relative simplicity; the small periodic repeat permits polyacetylene to be treated by sophisticated computational methods. -

Introduction to Polymer Solar Cells (Pscs)
2132-15 Winter College on Optics and Energy 8 - 19 February 2010 Introduction to Polymer Solar Cells (PSCs) K.S. Narayan Jawaharlal Nehru Centre for Advanced Scientific Research India K.S. Narayan Jawaharlal Nehru Centre for Advanced Scientific Research Bangalore,India [email protected] •Salubrious Weather < 22 °C > • Science and Technology Hub (S. Asia’s Si‐Valley) •Central Location to Historic and Natural Sites •Well connected • Culturally Vibrant•Verdant Environment, Academically Stimulating JAWAHARLAL NEHRU CENTRE FOR ADVANCED SCIENTIFIC RESEARCH An autonomous institution of Department of Science and Technology, established in 1989 Lecture 1 • Conducting Polymers, Semiconducting Polymers • Excitons in Semiconducting polymers • Diodes • Charge Separation… Bulk Hetrojunctions Kinetics‐rates‐branching factors Gaussian Model for Transport Lecture 2 • Jsc‐ Voc – Fill Factor Problems and Losses Bulk limiting factors Interfaces Mobility – Transport Symmetry – Aging‐degradation Lecture 3 • General Directions and Present Strategies • Recent Results from my laboratory Polymers: Structural variety … n Polyethylene H n Polypropylene CH3 n Polyvinyl chloride PVC Cl Polystyrene – Styrofoam, thermocole n Polyvinyl acetate - Fevicol n OAc • Insulators • Semiconductors • Conductors Pattern – Colours ‐ …… Conjugated Polymers n Polyacetylene n Polyphenylene vinylene S n Polythiophene Polypyrrole N n H N N N N n Polyaniline The 4 orbitals of a carbon atom in the form which allows it to couple to other atoms. When two atoms are brought together the wavefunctions which are in the plane overlap and couple. The attraction (stabilisation) introduced by the overlap. Polyacetylene • Despite the expectation of metallic behaviour, pristine polyacetylene was an insulator. Bond alternation due to Peierls distortion leads to the formation of a band gap between the valence and conduction band. -

The Discovery of Polyacetylene Film—The Dawning of an Era Of
REVIEWS OF MODERN PHYSICS, VOLUME 73, JULY 2001 Nobel Lecture: The discovery of polyacetylene film—the dawning of an era of conducting polymers* Hideki Shirakawa University of Tsukuba, Tsukuba, Ibaraki 305-8577, Japan (Published 20 September 2001) The Nobel Prize in Chemistry 2000 was awarded for our discovery and development of conducting polymers, but that discovery only happened after much work on polyacetylene. In this lecture, I would like to talk about the early investigations that preceded and eventually led to the discovery of chemical doping. I do hope my talk will be of use for you, the audience, to deepen your understanding of what had happened before and how we arrived at the idea of chemical doping. I. PROLOGUE difference between lengths of double and single bonds decreases with increasing conjugation and that all bonds It has been recognized for many years that a very long tend to be equal in length in an infinitely long polyene. linear conjugated polyene might have various interesting In other words, one would expect infinitely long one- properties, especially optical, electrical, and magnetic dimensional electrons to form a half filled band, or the properties. A polyene is an even number of methyne highest-occupied (HO) and the lowest-unoccupied (LU) (vCH-) groups, covalently bonded to form a linear car- -electron bands to merge with each other, leading to bon chain bearing one electron on each carbon atom. metallic behavior (Kuhn, 1948; Bayliss, 1952). In the Therefore the chemical structure of the polyene is best 1950s, however, it became theoretically clear that a poly- represented by the formula H(CHvCH)nH, where n ene with bond alternation is energetically more stable denotes the number of repeating units. -

Elimination Des Gaz
ELIMINATION DES GAZ IGC Doc 30/13/E Révision du Doc 30/10/E EUROPEAN INDUSTRIAL GASES ASSOCIATION AISBL AVENUE DES ARTS 3-5 • B-1210 BRUSSELS Tel : +32 2 217 70 98 • Fax: +32 2 219 85 14 E-mail : [email protected] • Internet : http://www.eiga.eu ELIMINATION DES GAZ RÉVISÉ PAR : Jean-Paul Barbier AIR LIQUIDE Giorgio Bissolotti SIAD Kevin Cleaver THE LINDE GROUP Esteban Elias AIR PRODUCTS Joachim Barbe MESSER GROUP Pierre Wolfs EIGA Déclaration Toutes les publications techniques éditées par EIGA ou sous son égide, et notamment ses codes de bonne pratique, les guides de procédures en matière de sécurité et toutes autres informations techniques contenues dans ces publications ont été élaborées avec le plus grand soin et établies avec les connaissances acquises des membres de EIGA ou de tiers à la date de leur publication. Elles n’ont la valeur juridique que de simples recommandations que les membres de EIGA ou les tiers ne sont pas tenus contractuellement de respecter: Elles ne peuvent faire l’objet vis-à-vis de quiconque, d’aucune garantie de la part d’EIGA. EIGA n’a ni le pouvoir, ni les moyens de vérifier que les codes de bonne pratique et les guides de procédures sont effectivement et correctement interprétés et appliqués par l’utilisateur qui engage seul sa responsabilité à cet égard. En conséquence, EIGA ne saurait en aucun cas être tenu pour responsable vis-à-vis de quiconque, de l’application par ses membres ou par toute autre personne, de ses codes de bonne pratique et guides de procédure. -

The Bromine Doping of Polyacetylene C.K
Available online at www.sciencedirect.com Physica A 321 (2003) 139–151 www.elsevier.com/locate/physa The bromine doping of polyacetylene C.K. Chiang Polymers Division, National Institute of Standards and Technology, Gaithersburg, MD 20899-8541, USA Abstract The original experiment of the bromine dopingof polyacetylene performedby Chiang andShi- rakawa is described. This simple, seminal experiment impacted physics and chemistry through the discovery of metallic conducting synthetic organic polymers and the veriÿcation and demonstra- tion of the doping concept in organic polymers. Although the molecular formula of polyacetylene is simple, this molecule exhibits complex behavior andfueling signiÿcant work not only in poly- mer science, but also in quantum physics. The soliton theories usedto describepolyacetylene’s simple but unique 1-D structure inspiredmany new quantum concepts for more complicated conducting polymers. The continuing study of model complex conducting polymers could lead to a better understanding of quantum electronic devices at the molecular level. c 2002 Elsevier Science B.V. All rights reserved. PACS: 72.82.Le; 82.35.Cd; 31.25 Keywords: Polyacetylene; Doping; Bromine; Chemistry Nobel Prize; Conducting polymer; 1-D conductor 1. Introduction The Nobel Prize in Chemistry 2000 was awarded to Alan J. Heeger, Alan G. Mac- DiarmidandHidekiShirakawa for their “Discovery andDevelopment of Conducting Polymers” [1–4]. In their Nobel lectures, the Laureates summarized over three decades of research. The ÿrst lecture given by Shirakawa, described his work on the devel- opment of polyacetylene chemistry. He ended his lecture with a story of the bromine doping of polyacetylene experiment [5]. Next, MacDiarmiddiscussedthechemistry of conducting polymers and recent development in plastic electronics [6]. -

A Review of Arsenic in Ambient Air in the UK
Report A Review of Arsenic in Ambient Air in the UK Prepared on behalf of : Department of the Environment, Transport and the Regions Scottish Executive The National Assembly for Wales February 2000 i Report A Review of Arsenic in Ambient Air in the UK Prepared By Richard Maggs - Principal Consultant Approved By Steve Moorcroft - Director Prepared for Department of the Environment, Transport and the Regions Scottish Executive The National Assembly for Wales February 2000 Our ref 305109 Your ref Document ref SSE/AQ/1465 ii Contents page 1. Introduction 7 2. Physical and Chemical Nature of Arsenic 7 2.1. Properties of Arsenic 7 2.2. Occurrence of Arsenic 8 3. The Atmospheric Chemistry of Arsenic 9 3.1. Particulate Arsenic 9 3.2. Vapour-phase Arsenic 10 4. UK Sources of Arsenic 12 4.1. Combustion Sources 12 4.2. Production Processes 13 4.2.1. Non-ferrous Metal Industry 14 4.2.2. Iron & Steel Industry 14 4.2.3. Sinter Plants 15 4.2.4. Non-ferrous Metal Mining 15 4.2.5. Waste Treatment and Disposal 15 4.2.6. Timber Industry 16 4.3. Emission Inventory Estimates 16 5. Air Quality Guidelines 20 6. Measurement Techniques 21 6.1. Sampling 21 6.2. Filter Media 21 6.3. Sample Preparation and analysis 22 6.3.1. Sample Preparation 23 6.3.2. Commonly Used Analytical Methods 24 6.4. Vapour-Phase Arsenic 25 6.5. Proposed Sampling Method 26 7. Monitoring in the UK 27 7.1. Rural Network Data 27 7.2. Urban Network Data 29 iii 7.3. -

Magnetic Properties of Polyacetylene Composites H
MAGNETIC PROPERTIES OF POLYACETYLENE COMPOSITES H. Thomann, L. Dalton, M. Galvin, G. Wnek, Y. Tomkiewicz To cite this version: H. Thomann, L. Dalton, M. Galvin, G. Wnek, Y. Tomkiewicz. MAGNETIC PROPERTIES OF POLYACETYLENE COMPOSITES. Journal de Physique Colloques, 1983, 44 (C3), pp.C3-313-C3- 316. 10.1051/jphyscol:1983361. jpa-00222713 HAL Id: jpa-00222713 https://hal.archives-ouvertes.fr/jpa-00222713 Submitted on 1 Jan 1983 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE Colloque C3, supplément au n°6, Tome 44, juin 1983 page C3-313 MAGNETIC PROPERTIES OF POLYACETYLENE COMPOSITES H. Thomaim*, L.R. Dalton*, M.E. Galvin**, G.E. Wnek**and Y. Tomkiewiez*** *Dept. of Chemistry, Univ. of Southern California, Los Angeles, CA 90089-0482, U.S.A. **Dept. of Materials Science and Engineering, M.I.T., Cambridge, MA 02139, U.S.A. ***IBM T.J. Watson Research Center, Yorktown Heights, NY 10598, U.S.A. Résumé - Des composites du polyéthylène basse densité, LDPE, et du trans-polyacétylène, t-(CH)x, préparés par polymérisation de l'acé tylène dans des films de LDPE imprégnés de catalyseur Ziegler-Natta, ont été étudiés par double résonance (ENDOR) et échos de spins élec troniques (ESE) et les résultats ont été comparés avec ceux obtenus dans les mêmes conditions sur des films de trans-polyacétylène. -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M.