(12) Patent Application Publication (10) Pub. No.: US 2017/0182285 A1 TYLER Et Al
Total Page:16
File Type:pdf, Size:1020Kb
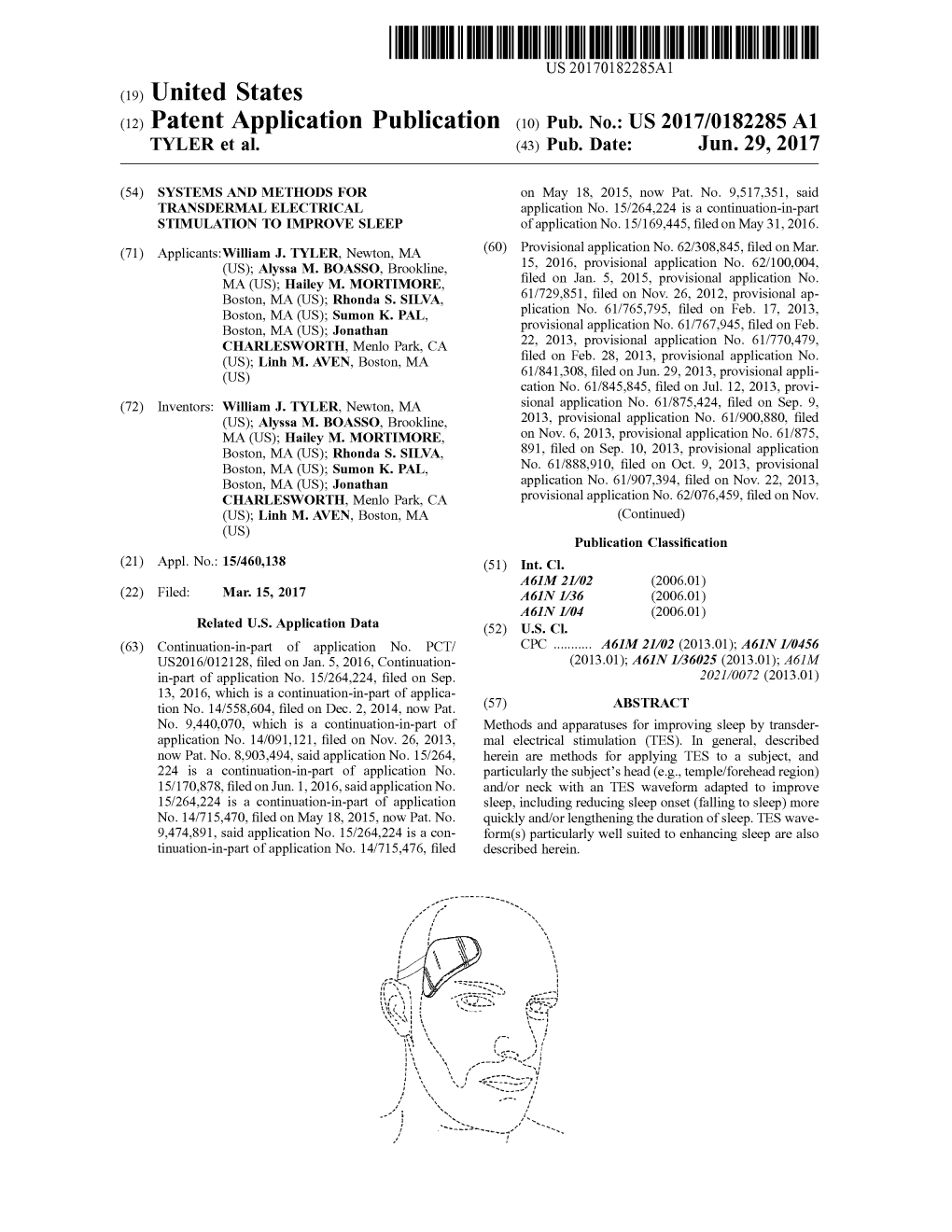
Load more
Recommended publications
-

Meet the Philosophers of Ancient Greece
Meet the Philosophers of Ancient Greece Everything You Always Wanted to Know About Ancient Greek Philosophy but didn’t Know Who to Ask Edited by Patricia F. O’Grady MEET THE PHILOSOPHERS OF ANCIENT GREECE Dedicated to the memory of Panagiotis, a humble man, who found pleasure when reading about the philosophers of Ancient Greece Meet the Philosophers of Ancient Greece Everything you always wanted to know about Ancient Greek philosophy but didn’t know who to ask Edited by PATRICIA F. O’GRADY Flinders University of South Australia © Patricia F. O’Grady 2005 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publisher. Patricia F. O’Grady has asserted her right under the Copyright, Designs and Patents Act, 1988, to be identi.ed as the editor of this work. Published by Ashgate Publishing Limited Ashgate Publishing Company Wey Court East Suite 420 Union Road 101 Cherry Street Farnham Burlington Surrey, GU9 7PT VT 05401-4405 England USA Ashgate website: http://www.ashgate.com British Library Cataloguing in Publication Data Meet the philosophers of ancient Greece: everything you always wanted to know about ancient Greek philosophy but didn’t know who to ask 1. Philosophy, Ancient 2. Philosophers – Greece 3. Greece – Intellectual life – To 146 B.C. I. O’Grady, Patricia F. 180 Library of Congress Cataloging-in-Publication Data Meet the philosophers of ancient Greece: everything you always wanted to know about ancient Greek philosophy but didn’t know who to ask / Patricia F. -

The Symbolism of Freemasonry
THE SYMBOLISM OF FREEMASONRY: ILLUSTBATING AND EXPLAINING gm ,irizurz and Qhilusuphy, its yzqmds, ' iitytlm, mul §ymhulK. PR* v" f 4l'=~*` ." » A 5 oi Q BY Q "Fo ALBERT G. MACKEY, M. D., Avmon or "Lexicon or Fam-:MAsoxnv," "'mx'r-noox or nmsomc .nm1sx>xzuDENcE," " cnnvrxc mAsom<Y," arc., arc. "Ea anim qua: scribuntur tria habers decent, uttlitalem przsenlnn, csrtum jinem, inezpugnabile fundamcntum." Cn.DANUs. NEW YORK: CLARK AND MAYNARD, 5 BARCLAY STREET. 1869. A26 "gms Entered, soeordlng to Act of Congress, ln the year 1809, by ALBERT G. MACKEY, In the Clerk'| Omce of the District Court ofthe District of South Carolina. lu|ntypedst¢lmBo|mn8tereotypeFoundry, No.l9Spr|n¢h.m. T0 GENERAL JOHN C. FREMONT. MY DEAR Sm: While any American might be proud of associating his name with that of one who has done so much to increase the renown of his country, and to enlarge the sum of human knowledge, this book is dedicated to you as a slight testimonial of regard for your personal char- acter, and in grateful recollection of acts of friendship. Yours very truly, A. G. MACKEY. 'Q _ "agggiafggffy .§a@&s§f» . éa W - |AR Y "ovrn¢ U NIVERSYYY ¢AL|r?;av\" PREFACE. OF the various modes of communicating instruction to the uninformed, the masonic student is particularly interested ln two; namely, the instruction by legends and that by symbols. It is to these two, almost exclusively, that he is indebted for all that he knows, and for all that he can know, of the philosophic system which is taught in the institution. -

Recorded Jazz in the 20Th Century
Recorded Jazz in the 20th Century: A (Haphazard and Woefully Incomplete) Consumer Guide by Tom Hull Copyright © 2016 Tom Hull - 2 Table of Contents Introduction................................................................................................................................................1 Individuals..................................................................................................................................................2 Groups....................................................................................................................................................121 Introduction - 1 Introduction write something here Work and Release Notes write some more here Acknowledgments Some of this is already written above: Robert Christgau, Chuck Eddy, Rob Harvilla, Michael Tatum. Add a blanket thanks to all of the many publicists and musicians who sent me CDs. End with Laura Tillem, of course. Individuals - 2 Individuals Ahmed Abdul-Malik Ahmed Abdul-Malik: Jazz Sahara (1958, OJC) Originally Sam Gill, an American but with roots in Sudan, he played bass with Monk but mostly plays oud on this date. Middle-eastern rhythm and tone, topped with the irrepressible Johnny Griffin on tenor sax. An interesting piece of hybrid music. [+] John Abercrombie John Abercrombie: Animato (1989, ECM -90) Mild mannered guitar record, with Vince Mendoza writing most of the pieces and playing synthesizer, while Jon Christensen adds some percussion. [+] John Abercrombie/Jarek Smietana: Speak Easy (1999, PAO) Smietana -

NGA | 2012 Annual Report
NA TIO NAL G AL LER Y O F A R T 2012 ANNUAL REPort 1 ART & EDUCATION Diana Bracco BOARD OF TRUSTEES COMMITTEE Vincent J. Buonanno (as of 30 September 2012) Victoria P. Sant W. Russell G. Byers Jr. Chairman Calvin Cafritz Earl A. Powell III Leo A. Daly III Frederick W. Beinecke Barney A. Ebsworth Mitchell P. Rales Gregory W. Fazakerley Sharon P. Rockefeller Doris Fisher John Wilmerding Juliet C. Folger Marina Kellen French FINANCE COMMITTEE Morton Funger Mitchell P. Rales Lenore Greenberg Chairman Frederic C. Hamilton Timothy F. Geithner Richard C. Hedreen Secretary of the Treasury Teresa Heinz Frederick W. Beinecke John Wilmerding Victoria P. Sant Helen Henderson Sharon P. Rockefeller Chairman President Benjamin R. Jacobs Victoria P. Sant Sheila C. Johnson John Wilmerding Betsy K. Karel Linda H. Kaufman AUDIT COMMITTEE Robert L. Kirk Frederick W. Beinecke Leonard A. Lauder Chairman LaSalle D. Leffall Jr. Timothy F. Geithner Secretary of the Treasury Edward J. Mathias Mitchell P. Rales Diane A. Nixon John G. Pappajohn Sharon P. Rockefeller Frederick W. Beinecke Mitchell P. Rales Victoria P. Sant Sally E. Pingree John Wilmerding Diana C. Prince Robert M. Rosenthal TRUSTEES EMERITI Roger W. Sant Robert F. Erburu Andrew M. Saul John C. Fontaine Thomas A. Saunders III Julian Ganz, Jr. Fern M. Schad Alexander M. Laughlin Albert H. Small David O. Maxwell Michelle Smith Ruth Carter Stevenson Benjamin F. Stapleton Luther M. Stovall Sharon P. Rockefeller John G. Roberts Jr. EXECUTIVE OFFICERS Ladislaus von Hoffmann Chief Justice of the Diana Walker United States Victoria P. Sant President Alice L. -

AVANT Volume III, Number 1/2012 187
AVANT Volume III, Number 1/2012 www.avant.edu.pl/en 187 188 Studies on Musical Practice Matthew Shipp by Phil Freeman I’ve written about Matt Shipp almost annually for over a decade. I’ve reviewed his albums, interviewed him, profiled him at length in Burning Ambulance magazine, and discussed the work of other musicians with him in person, on the phone, and by email. I am not an objective observer when it comes to Matt Shipp or his work. I consider him a friend. Despite this blurring of the line between critical appreciation and personal friend- liness, I think it’s safe to say that Matt’s doing the best work of his career right now, that his voice on the piano has matured into something powerful, unique, and al- most instantly recognizable. He even manages to make the piano trio sound inter- esting, something I might otherwise have argued was impossible in 2012. Matt’s most recent trio album, Elastic Aspects , is a perfect example of how he’s breaking the rules governing the jazz piano trio while still creating music of great, and accessible, beauty. He grants a tremendous amount of space to bassist Michael Bisio; indeed, there are times when the piano is almost totally absent, when it’s just bass, or just bass and drums, and yet the music retains the feeling of suspense, of continuous forward movement and multiple co-participants’ simultaneous concen- tration on a single shared idea that is the heart of jazz. He has long abandoned many of the clichés of the avant-garde, having arrived at a sound that is far more indebted to post-bebop piano players of the 1950s and early 1960s than to so-called free players of the ’60s and beyond like Cecil Taylor, Bobby Few or Dave Burrell, though he’ll happily praise those men, too. -

AZTEC ARCHITECTURE by MANUEL AGUILAR-MORENO, Ph.D
AZTEC ARCHITECTURE by MANUEL AGUILAR-MORENO, Ph.D. PHOTOGRAPHY: FERNANDO GONZÁLEZ Y GONZÁLEZ AND MANUEL AGUILAR-MORENO, Ph.D. DRAWINGS: LLUVIA ARRAS, FONDA PORTALES, ANNELYS PÉREZ, RICHARD PERRY AND MARIA RAMOS. TABLE OF CONTENTS INTRODUCTION Symbolism TYPES OF ARCHITECTURE General Construction of Pyramid-Temples Temples Types of pyramids Round Pyramids Twin Stair Pyramids Shrines (Adoratorios ) Early Capital Cities City-State Capitals Ballcourts Aqueducts and Dams Markets Gardens BUILDING MATERIALS AND TECHNIQUES THE PRECINCT OF TENOCHTITLAN Introduction Urbanism Ceremonial Plaza (Interior of the Sacred Precinct) The Great Temple Myths Symbolized in the Great Temple Construction Stages Found in the Archaeological Excavations of the Great Temple Construction Phase I Construction Phase II Construction Phase III Construction Phase IV Construction Phase V Construction Phase VI Construction Phase VII Emperor’s Palaces Homes of the Inhabitants Chinampas Ballcourts Temple outside the Sacred Precinct OTHER CITIES Tenayuca The Pyramid Wall of Serpents Tomb-Altar Sta. Cecilia Acatitlan The Pyramid Teopanzolco Tlatelolco The Temple of the Calendar Temple of Ehecatl-Quetzalcoatl Sacred Well Priests’ Residency The Marketplace Tetzcotzinco Civic Monuments Shrines Huexotla The Wall La Comunidad (The Community) La Estancia (The Hacienda) Santa Maria Group San Marcos Santiago The Ehecatl- Quetzalcoatl Building Tepoztlan The Pyramid-Temple of Tepoztlan Calixtlahuaca Temple of Ehecatl-Quetzalcoatl The Tlaloc Cluster The Calmecac Group Ballcourt Coatetelco Malinalco Temple I (Cuauhcalli) – Temple of the Eagle and Jaguar Knights Temple II Temple III Temple IV Temple V Temple VI Figures Bibliography INTRODUCTION Aztec architecture reflects the values and civilization of an empire, and studying Aztec architecture is instrumental in understanding the history of the Aztecs, including their migration across Mexico and their re-enactment of religious rituals. -

The Temple of Quetzalcoatl and the Cult of Sacred War at Teotihuacan
CHAPTER 7 TheTempleofQuetzalcoatlandthe Cultof Sacred Warat Teotihuacan The Temple of Quetzalcoatl at Teotihuacan has been the source of startling archaeological discoveries since the early portion of this century. Beginning in 1918, excavations by Manuel Gamio revealed an elaborate and beautifully preserved facade underlying later construc- tion. Although excavations were performed intermittently during the subsequent decades, some of the most important discoveries have occurred during the last several years. Recent investigations have revealed mass dedicatory burials in the foundations of the Temple of Quetzalcoatl (Sugiyama 1989a; Cabrera Castro et al. 1988); at the time of this writing, more than eighty individuals have been discovered interred in the foundations of the pyramid. Sugiyama (1989a) persuasively argues that many of the individuals appear to be either war- riors or dressed in the office of war. The archaeological investigations by Cabrera, Sugiyama, and Cowgill are ongoing, and to comment extensively on the implications of their work would be both premature and presumptuous. Nonetheless, the recent excavations have placed an entirely new light on the significance of the Temple of Quetzalcoatl and its remarkable sculptural format. In this study, I will be concerned with the iconographic meaning of the Temple of Quetzalcoatl facade. In recent work, I noted that the temple facade represents serpents passing through a facade of circular mirrors (Taube 1986, 1988e). Two forms of serpents are present, Quetzalcoatl and an ancestral form of the Xiuhcoatl. In this respect, the Temple of Quetzalcoatl facade may be compared to the Postclassic wind temple of Ehecatl-Quetzalcoatl, which also appears To cite this chapter: with mirrors and serpents (Taube 1986). -

Julian Cowley on Kissing Time, Crude Vigour, and a Chuntering Escalator
solo journey of his own. Around three which wades in the EST/ECM pool, perks minutes in, the rest of the group sneak into up substantially when de Looze switches New books from Repeater. position for a chamber music-like exchange from piano to Fender Rhodes. Boehme’s big, of soft focus phrases. latterday Charlie Haden sound adds heart. Kate Gentile Meridian Trio Mannequins Triangulum Skirl CD/DL Clean Feed CD Six of the 13 compositions on drummer Meridian Trio is a meeting place for three Kate Gentile’s first album as a leader are Chicago players with solid reputations as under three minutes; four others last leaders: alto saxophonist Nick Mazzarella, Julian Cowley on between eight and 13 minutes. The group bassist Matt Ulery and drummer Jeremy – saxophonist Jeremy Viner, Matt Mitchell Cunningham. Together, they create music kissing time, on piano and Prophet synth, Adam Hopkins that recalls Ornette Coleman’s 1960s trio on bass – are adept at the sort of tinkling, with David Izenzon and Charles Moffett crude vigour, and squawking post-Dolphy, post-Braxton, post- without imitating it. Cunningham takes an a chuntering Berne chamber jazz that flows from every epic, suspenseful solo on the title piece rehearsal studio in Brooklyn nowadays. But that sounds like Max Roach warming up escalator UNDER MY THUMB Rhian E Jones the synth, Gentile’s haunting vibraphone for the main event; Mazzarella’s playing ISBN: 9781910924617 and the short interludes make Mannequins on “Ringdown” has a Coltrane-ish cry, and Eli Davies more than a collection of wilfully unresolved supported by Ulery’s booming single notes. -

Political Strategies in Pre-Columbian Mesoamerica
Political Strategies in Pre-Columbian Mesoamerica EDITED BY Sarah Kurnick and Joanne Baron Political Strategies in Pre-Columbian Mesoamerica Political Strategies in Pre-Columbian Mesoamerica EDITED BY Sarah Kurnick and Joanne Baron UNIVERSITY PRESS OF COLORADO Boulder © 2016 by University Press of Colorado Published by University Press of Colorado 5589 Arapahoe Avenue, Suite 206C Boulder, Colorado 80303 All rights reserved Printed in the United States of America The University Press of Colorado is a proud member of Association of American University Presses. The University Press of Colorado is a cooperative publishing enterprise supported, in part, by Adams State University, Colorado State University, Fort Lewis College, Metropolitan State University of Denver, Regis University, University of Colorado, University of Northern Colorado, Utah State University, and Western State Colorado University. ∞ This paper meets the requirements of the ANSI/NISO Z39.48-1992 (Permanence of Paper). ISBN: 978-1-60732-415-7 (cloth) ISBN: 978-1-60732-416-4 (ebook) Library of Congress Cataloging-in-Publication Data Political strategies in pre-Columbian Mesoamerica / edited by Sarah Kurnick and Joanne Baron. pages cm ISBN 978-1-60732-415-7 (hardback) — ISBN 978-1-60732-416-4 (ebook) 1. Indians of Mexico—Antiquities. 2. Indians of Central America—Antiquities. 3. Indians of Mexico—Politics and government. 4. Indians of Central America—Politics and government. 5. Authority—Political aspects—Mexico—History—To 1500. 6. Authority—Political aspects— Central America—History—To 1500. 7. Social archaeology—Mexico. 8. Social archaeology— Central America. 9. Ethnoarchaeology—Mexico. 10. Ethnoarchaeology—Central America. I. Kurnick, Sarah. II. Baron, Joanne. F1219.3.P7P66 2015 972’.01—dc23 2015010767 26 25 24 23 22 21 20 19 18 17 10 9 8 7 6 5 4 3 2 1 Cover illustrations: detail from the Codex Zouche-Nuttall © the Trustees of the British Museum (foreground ); detail of the Lienzo de Ocotepec, courtesy, Vanderbilt University Publications in Anthropology (background ). -

Elixir Journal
50763 Ramiro Sofronie / Elixir Social Studies 118C (2018) 50763-50779 Available online at www.elixirpublishers.com (Elixir International Journal) Social Studies Elixir Social Studies 118C (2018) 50763-50779 Homo Astralis Ramiro Sofronie Bucharest, Romania. ARTICLE INFO ABSTRACT Article history: The paper deals with the fate of homo gravitas. As an archetype of the existing humans, Received: 13 April 2018; homo gravitas is mirroring in Heraclitus‟ universe like into its whole. In spite of this Received in revised form: privileged astral position, homo gravitas is experiencing trouble with its own life at 6 May 2018; home, on the earth and behaves cruelly to its environment. This paper brings proof that Accepted: 17 May 2018; all trouble is due to some disconnections occurring with its gravitational and energetic ties. Some of the damage lasts for a long time, but most has been produced during the Keywords former cultures. Those disconnections disturb what Paracelsus called the inner harmony Awareness, of humans and psychic disorders result. They disclose the existing conflicts between the Balance, body and the mind. It was evaluated that 25% of total population is seriously affected by Disconnection, the disease called here schizophrenia astralis. It is not an infecting disease, but its danger Identity, increases with population growth. By raising awareness and eliminating its causes with Inner-harmony, equanimity, schizophrenia astralis can be eradicated before it is too late. Then, with a Mutual-attraction, well-balanced body-mind couple, homo gravitas automatically becomes homo astralis. Schizophrenia-astralis. Anyway, there is no longer need to emigrate on other planets or meet aliens. -

The J. Paul Getty Museum Journal Volume 5 1977
THE J. PAUL GETTY MUSEUM JOURNAL VOLUME 5 1977 The J. Paul Getty Museum Malibu, California Volume 5 Jití Freí, Editor Published by the J. Paul Getty Museum, Malibu, California © 1978 The J. Paul Getty Museum All rights reserved ISBN 0-89236-000-3 Produced in Chicago by Ares Publishers, Inc. Abbreviation: Getty MJ This issue includes for the first time contributions dealing with conservation and related matters; thus, it is an appropriate tribute to the memory of David Rinne, who headed the conservation of antiquities in the J. Paul Getty Museum from the Fall of 1973 to the end of 1976. The Editor wishes to thank Summa Galleries Inc. of Beverly Hills for a grant in Mr. Rinne's memory which allowed us to expand this volume. A note on Mr. Rinne's conservation of ancient marbles will appear in Volume 7. Most of the photographs of the pieces in the J. Paul Getty Museum were taken by Donald A. Hull, the Museum photographer. For a forthcoming issue the curators of the Museum are preparing contributions on J. Paul Getty, our late founder, as a collector. Editor D M DAVID RINNE CVIVS ARS MONVMENTA GETTIANA RESTITVIT VIXIT ANN XXXV AB AMICIS DEFLETVS CONTENTS GREEK AND ROMAN SCULPTURE PAINTINGS E in Metopenkopfvom Parthenon, M. Weber 5 Two Newly Discovered Ceiling Paintings by B.B. Fredericksen Ein Junglingshopf in Matibu, F. Brommer 13 Simon Vouet, 95 J. Freí 17 Seven Paintings from the Fesch Collection, A Youth from the Parthenon?, M. Wynne 101 Ein Grossgriechischer Ahrolith imJ. Paul G. Olbrich 21 Metamorphoses of the Grimani "Vitellius", Getty Museum, S. -

A Reconstruction of the Settings for Three Operas Designed by Filippo Juvarra in Rome, 1710-1712
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1973 A Reconstruction of the Settings for Three Operas Designed by Filippo Juvarra in Rome, 1710-1712. Thomas Charles Tews Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Tews, Thomas Charles, "A Reconstruction of the Settings for Three Operas Designed by Filippo Juvarra in Rome, 1710-1712." (1973). LSU Historical Dissertations and Theses. 2576. https://digitalcommons.lsu.edu/gradschool_disstheses/2576 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image.