Alberta Pharmaceutical Strategy: Stakeholder Consultation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Local Authorities Pension Plan Cost of Living
SENIORS UNITED NOW LOCAL AUTHORITIES PENSION PLAN COST OF LIVING ADVOCACY PROJECT IDENTIFY THE ISSUE: Alberta pensioners annual cost of living adjustment is currently based on only 60% of the Canadian consumer price index. For the average government pensioner this calculated to a 0.96% increase, which in dollars amounts to about a $10.00 monthly increase, resulting in a further fall behind of the real inflation rate. The Alberta Provincial Government promised cost of living adjustments to be comparable to the C.P.I. but continues to break this promise. A recent press release from the Local Authorities Pension Plan indicates the total elimination of the Cost of Living Adjustment! COST OF LIVING ADJUSTMENT STANDARDS SET BY OTHER PROVINCES: Following is a summary, obtained from Google, which outlines the COLA adjustment standards implemented by other Provincial Governments; British Columbia, over the last 25 years (except for one year) has provided COLA adjustments which provides increases at 100% of the Canadian Consumer Price Index. Manitoba, COLA is based on 2/3 of the C.P.I. Ontario, appears to be 100% of the C.P.I. Quebec, pensioners over the age of 65 receive 100% of the C.P.I. Newfoundland/Labrador, COLA is adjusted October of each year to 60% of the C.P.I. New Brunswick, has a “Shared Risk Pension Model”, as pension returns increase the higher the COLA is adjusted. Nova Scotia, if superannuation fund is insufficient , the Minister of Finance shall pay the shortfall out of General Revenue. Year 2011 to 2015 COLA is indexed at 1.25%. -

University of Lethbridge Alumnus Manwar Khan to Hold Anti-‐Bullying
For Immediate Release — Wednesday, August 12, 2015 University of Lethbridge alumnus Manwar Khan to hold Anti-Bullying Rally for victims of bullying and violence What: Do Not Be a Bystander Anti-Bullying Rally When: Saturday, August 15, 11:30 a.m. Where: Calgary City Hall – Municipal Plaza space Edmonton-based activist and University of Lethbridge alumnus Manwar Khan (BSc ’07), a father of twins, is holding a rally in Calgary at the Calgary City Hall as he continues to campaign across the province against bullying and violence. “We will be gathering in front of City Hall to show our support for every known and unknown victim of bullying and violence in Alberta,” says Khan, who witnessed a violent attack on an Edmonton LRT train in December 2012. It led to the death of one man and the incarceration of another. Khan invites everyone to gather at City Hall at 11:30 a.m. to rally in support of those who have been affected by violence and bullying. He is also asking people who are not able to attend the rally to leave their porch lights on from 6 to 7 p.m. on August 15, 2015 to show their support for the stand against violence and bullying. Khan established a series of anti-bullying rallies in 2013 and 2014, holding events in Edmonton, Calgary, Lethbridge and Airdrie. He has vowed to continue to represent victims of bullying and to try and stem the onset of violence so that it does not persist in society. “I believe firmly that it is a matter of great importance to teach our children that bullying is simply not acceptable in our society if we are to achieve safety and happiness for all,” he says. -
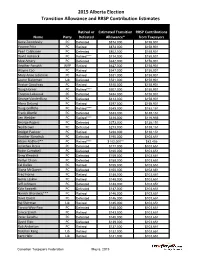
2015 Alberta Election Transition Allowance and RRSP Contribution Estimates
2015 Alberta Election Transition Allowance and RRSP Contribution Estimates Retired or Estimated Transition RRSP Contributions Name Party Defeated Allowance* from Taxpayers Gene Zwozdesky PC Defeated $874,000 $158,901 Yvonne Fritz PC Retired $873,000 $158,901 Pearl Calahasen PC Defeated $802,000 $158,901 David Hancock PC Retired**** $714,000 $158,901 Moe Amery PC Defeated $642,000 $158,901 Heather Forsyth WRP Retired $627,000 $158,901 Wayne Cao PC Retired $547,000 $158,901 Mary Anne Jablonski PC Retired $531,000 $158,901 Laurie Blakeman Lib Defeated $531,000 $158,901 Hector Goudreau PC Retired $515,000 $158,901 Doug Horner PC Retired**** $507,000 $158,901 Thomas Lukaszuk PC Defeated $484,000 $158,901 George VanderBurg PC Defeated $413,000 $158,901 Alana DeLong PC Retired $397,000 $158,901 Doug Griffiths PC Retired**** $349,000 $152,151 Frank Oberle PC Defeated $333,000 $138,151 Len Webber PC Retired**** $318,000 $116,956 George Rogers PC Defeated $273,000 $138,151 Neil Brown PC Defeated $273,000 $138,151 Bridget Pastoor PC Retired $238,000 $138,151 Heather Klimchuk PC Defeated $195,000 $103,651 Alison Redford** PC Retired**** $182,000** $82,456 Jonathan Denis PC Defeated $177,000 $103,651 Robin Campbell PC Defeated $160,000 $103,651 Greg Weadick PC Defeated $159,000 $103,651 Verlyn Olson PC Defeated $158,000 $103,651 Cal Dallas PC Retired $155,000 $103,651 Diana McQueen PC Defeated $150,000 $103,651 Fred Horne PC Retired $148,000 $103,651 Genia Leskiw PC Retired $148,000 $103,651 Jeff Johnson PC Defeated $148,000 $103,651 Kyle Fawcett -

Orange Chinook: Politics in the New Alberta
University of Calgary PRISM: University of Calgary's Digital Repository University of Calgary Press University of Calgary Press Open Access Books 2019-01 Orange Chinook: Politics in the New Alberta University of Calgary Press Bratt, D., Brownsey, K., Sutherland, R., & Taras, D. (2019). Orange Chinook: Politics in the New Alberta. Calgary, AB: University of Calgary Press. http://hdl.handle.net/1880/109864 book https://creativecommons.org/licenses/by-nc-nd/4.0 Attribution Non-Commercial No Derivatives 4.0 International Downloaded from PRISM: https://prism.ucalgary.ca ORANGE CHINOOK: Politics in the New Alberta Edited by Duane Bratt, Keith Brownsey, Richard Sutherland, and David Taras ISBN 978-1-77385-026-9 THIS BOOK IS AN OPEN ACCESS E-BOOK. It is an electronic version of a book that can be purchased in physical form through any bookseller or on-line retailer, or from our distributors. Please support this open access publication by requesting that your university purchase a print copy of this book, or by purchasing a copy yourself. If you have any questions, please contact us at [email protected] Cover Art: The artwork on the cover of this book is not open access and falls under traditional copyright provisions; it cannot be reproduced in any way without written permission of the artists and their agents. The cover can be displayed as a complete cover image for the purposes of publicizing this work, but the artwork cannot be extracted from the context of the cover of this specific work without breaching the artist’s copyright. COPYRIGHT NOTICE: This open-access work is published under a Creative Commons licence. -

Premier Promotes Verlyn Olson and Greg Weadick to Cabinet Cal Dallas Becomes the New Parliamentary Assistant to Finance
February 17, 2011 Premier promotes Verlyn Olson and Greg Weadick to cabinet Cal Dallas becomes the new Parliamentary Assistant to Finance Edmonton... Premier Ed Stelmach announced today that Wetaskiwin-Camrose MLA Verlyn Olson, QC, has been named Minister of Justice and Attorney General, and Lethbridge West MLA Greg Weadick has been named Minister of Advanced Education and Technology. “I’m pleased to welcome Verlyn and Greg to the cabinet table,” said Premier Stelmach. “Verlyn and Greg bring the necessary talent and experience - Greg as a parliamentary assistant and Verlyn as a long-time member of the bar - to complete our cabinet team. Our cabinet will continue to provide the steady leadership required right now to continue building a better Alberta.” Premier Stelmach also named a new Parliamentary Assistant to the Minister of Finance and Enterprise. “I’m pleased that Red Deer South MLA Cal Dallas, who had been serving as the Parliamentary Assistant in Environment, will take over this important role and work closely with Finance Minister Lloyd Snelgrove,” said the Premier. Premier Stelmach also announced changes to committee memberships. Joining the Agenda and Priorities Committee are Sustainable Resource Development Minister Mel Knight, Children and Youth Services Minister Yvonne Fritz and Agriculture and Rural Development Minister Jack Hayden. New members of Treasury Board are Len Webber, Minister of Aboriginal Relations, Heather Klimchuk, Minister of Service Alberta, and Naresh Bhardwaj, MLA for Edmonton-Ellerslie. The new Cabinet members will be sworn in Friday, February 18 at 8:30 a.m. at Government House. Lloyd Snelgrove was sworn in as Minister of Finance and Enterprise on January 31. -

Naughty and Nice to Taxpayers List’ 2013
‘Naughty and Nice to Taxpayers List’ 2013 Naughty Alison Redford: Premier Redford flies around the world more than Santa Claus and gives out more free gifts paid for by debt than the big man in red could ever hope to, using mere elves. Total expense: $82,872 Notable airfare expense claims: o $7,875 to New Brunswick o $6,092 to Chicago o $5,927 to Toronto Notable hotel expense claims: o $876/night in Washington (x2) o $772/night in New York (x3) o $635/night in Toronto (x2) o $649/night in Ottawa (x2) Notable meal & hospitality claims o $22 coffees in Washington o $31 hamburger in Washington Doug Horner: Government debt is sort of like making the kids pay Santa for mom and dad’s presents. Since Doug is leaving behind debt for the little ones, Santa is likely to leave behind a lump of coal for him this year. Large debt and deficit; Even the Auditor General can’t figure out how to make sense out of his books; Initially banning the CTF from the budget lockup. Thomas Lukaszuk: Less naughty than last year, but Minister Lukaszuk sure does love flying to Calgary any chance that the can get. No driving for him. He even managed to find an excuse to be in Calgary on ‘government business’ for five days during Stampede. The last time Santa spent five days at Stampede, the elves fell behind on Cabbage Patch Kids and Mrs. Claus made him sleep on the couch for a week. Add to this his threat to raise beer taxes, and he really had no chance of making the Nice List this year. -

Len Webber Named New Minister of International and Intergovernmental Relations Premier Ed Stelmach Also Appoints New Parliamentary Assistants
September 16, 2009 Len Webber named new Minister of International and Intergovernmental Relations Premier Ed Stelmach also appoints new Parliamentary Assistants Edmonton... Premier Ed Stelmach has announced that Calgary-Foothills MLA Len Webber will be sworn in as the new Minister of International and Intergovernmental Relations by Lieutenant Governor Norman L. Kwong on Thursday, September 17. “I’m very pleased to have Len at the cabinet table,” said Premier Stelmach. “He has performed exceptionally well as the Parliamentary Assistant for Energy, and I’m happy to have him join cabinet in the crucial portfolio of International and Intergovernmental Relations.” Premier Stelmach also named Calgary-Egmont MLA Jonathan Denis as the new Parliamentary Assistant for Energy. Cardston-Taber-Warner MLA Broyce Jacobs becomes the Parliamentary Assistant for Agriculture and Rural Development (ARD). And Battle River-Wainwright MLA Doug Griffiths moves from his role as the Parliamentary Assistant in ARD to become the Parliamentary Assistant for the Department of Solicitor General and Public Security. Premier Stelmach also announced the following Cabinet committee changes to fill existing vacancies: Advanced Education and Technology Minister Doug Horner has been named the new vice-chair of the Agenda and Priorities Committee, while Culture and Community Spirit Minister Lindsay Blackett has been named to the Agenda and Priorities Committee. Athabasca-Redwater MLA Jeff Johnson and Airdrie-Chestermere MLA Rob Anderson have been named to Treasury Board. Also, Minister of Justice and Attorney General Alison Redford and Employment and Immigration Minister Hector Goudreau have been added to the roster of Deputy Government House Leaders. -30- Assignment Editors: Media are invited to attend the swearing-in of new International and Intergovernmental Relations Minister Len Webber. -

SPRING Convention15
‘ SPRING convention15 Convention Highlights March 16-18, 2015 March 16 - Trade Show TRADE SHOW With over 140 booths, this year’s AAMDC Spring Trade Show was filled with products, services and ideas for our members to discover. The diverse set of exhibitors included everything from library systems, cellular services and census options to wetland conservation and auto parts. Many exhibitors brought in equipment for attendees to see firsthand including graders, tires, street sweepers and traffic signs. Every year the Trade Show is a great opportunity for AAMDC members to see the products they are purchasing up close and this year was no different. As the Trade Show came to a close, members and suppliers were invited to the AAMDC Aggregated Business Services Open House sponsored by Fortis Alberta to meet and engage with Aggregated Business Services staff members. Mike Pashak from Fortis Alberta was on hand to draw for the Trade Show grand prize of an Apple iPad. AAMDC Aggregated Business Services (ABS), which is comprised of the Trade Division, PFA Canada, and Jubilee Insurance Agencies, continues to focus on delivering excellent member services. This year, we have increased our staff compliment by adding Carolyn Boyle as a Manager of Client Relations, and Dayna Johnson as a Risk Management Advisor. These additions coupled with improving technology platforms in Trade and PFA Canada, represent our commitment to adding value to the ABS offerings. This year, the Trade Division has again negotiated deeper discounts, Jubilee Insurance has been able complete its appraisal program, and PFA has grown significantly in several provinces. These achievements have resulted in one of the strongest years that ABS has ever had. -

Alberta Heritage Savings Trust Fund
Twenty-Seventh Legislature Second Session Standing Committee on the Alberta Heritage Savings Trust Fund ANNUAL REPORT 2008-2009 COMMITTEES OF THE LEGISLATIVE ASSEMBLY STANDING COMMITTEE ON THE ALBERTA HERITAGE SAVINGS TRUST FUND Room 801 Legislature Annex, 9718 - 107 Street Edmonton, Alberta, T5K 1E4 Tel: (780) 427-1348; Fax: (780) 427-5688 email: [email protected] Chair: Members: HEATHER FORSYTH, MLA LAURIE BLAKEMAN, MLA ART JOHNSTON, MLA Calgary-Fish Creek Edmonton-Centre Calgary-Hays Deputy Chair: ROBIN CAMPBELL, MLA DARSHAN KANG, MLA West Yellowhead Calgary-McCall DOUG ELNISKI, MLA Edmonton-Calder ALANA DeLONG, MLA HUGH MacDONALD, MLA Calgary-Bow Edmonton-Goldbar JONATHAN DENIS, MLA Calgary-Egmont September 2009 Honourable Ken Kowalski Speaker of the Legislative Assembly of the Province of Alberta Dear Speaker Kowalski: The Standing Committee on the Alberta Heritage Savings Trust Fund has the honour to submit its report for the 2008-2009 fiscal year. Sincerely, [Original signed by the chair] Heather Forsyth, MLA Chair, Standing Committee on the Alberta Heritage Savings Trust Fund c. Dr. W.J. David McNeil Clerk of the Legislative Assembly of Alberta MEMBERS 2008-2009 Fiscal Year CURRENT MEMBERS HEATHER FORSYTH, Chair HEATHER FORSYTH, Chair MLA, Calgary-Fish Creek (PC) MLA, Calgary-Fish Creek (PC) DOUG ELNISKI, Deputy Chair DOUG ELNISKI, Deputy Chair MLA, Edmonton-Calder (PC) MLA, Edmonton-Calder (PC) LAURIE BLAKEMAN LAURIE BLAKEMAN MLA, Edmonton-Centre (Lib) MLA, Edmonton-Centre (Lib) ALANA DeLONG ROBIN CAMPBELL MLA, Calgary-Bow -

The Alberta Gazette
The Alberta Gazette Part I Vol. 110 Edmonton, Saturday, November 29, 2014 No. 22 PROCLAMATION [GREAT SEAL] CANADA PROVINCE OF ALBERTA Donald S. Ethell, Lieutenant Governor. ELIZABETH THE SECOND, by the Grace of God, of the United Kingdom, Canada, and Her Other Realms and Territories, QUEEN, Head of the Commonwealth, Defender of the Faith P R O C L A M A T I O N To all to Whom these Presents shall come G R E E T I N G Kim Armstrong Deputy Attorney General WHEREAS section 24 of the Tobacco Reduction Amendment Act, 2013 provides that that Act comes into force on Proclamation; and WHEREAS it is expedient to proclaim certain provisions of the Tobacco Reduction Amendment Act, 2013 in force: NOW KNOW YE THAT by and with the advice and consent of Our Executive Council of Our Province of Alberta, by virtue of the provisions of the said Act hereinbefore referred to and of all other power and authority whatsoever in Us vested in that behalf, We have ordered and declared and do hereby proclaim the following provisions of the Tobacco Reduction Amendment Act, 2013 in force on the following dates: (a) on November 13, 2014, sections 1, 2, 3(a) and (b), 4(b), 5, 8(b), 10, 11(a), 12 to 18, and 19(a); section 19(d) to the extent that it enacts section 9(1)(e.1) to (e.3) of the Tobacco Reduction Act; section 19(f) to the extent that it enacts section 9(1)(g) and (g.1) of the Tobacco Reduction Act; and sections 21 and 23; (b) on June 1, 2015, sections 9, 11(b) and 19(e). -

Member's Public Disclosure Statement
MEMBER'S PUBLIC DISCLOSURE STATEMENT Pursuant to Conflicts of Interest Act, Chapter C-23, RSA 2000 As at April 1, 2014 (including material amendment changes to November 30, 2014) NAME OF MEMBER: Mike Allen CONSTITUENCY: Fort McMurray-Wood Buffalo Not Applicable Applicable Form 1 Statement of Member X Form 2 Statement of Member's Spouse/ Adult Interdependent Partner X Form 3 Statement of Member's Minor Children X Form 4 Statement of Private Corporations X NOTE: Under Section 14 of the Conflicts of Interest Act, Chapter C-23, RSA 2000: A public disclosure statement shall identify (a) the assets, liabilities, financial interests, and sources of income, (b) the fees, gifts, or benefits approved for retention under section 7(2)(b), and (c) any travel accepted under section 7.1, as disclosed in a Member's private disclosure statement but shall not state the amount or value of those items. Excluded from a public disclosure statement are the following: (a) assets, liabilities, or interests having a value of less than $10,000; (b) a source of income of less than $5,000 per year; (c) information identifying a home or recreational property occupied by the Member or one of the Member's family; (d) personal property that the Member, the Member’s spouse or adult interdependent partner or one of the Member’s family uses primarily for transportation, household, educational, recreational, social or aesthetic purposes; (e) unpaid taxes, except property taxes under the Municipal Government Act and taxes under the School Act; and (f) support obligations. -

Alberta Pharmaceutical STRATEGY
Alberta Pharmaceutical STRATEGY PB table of contents .0 Introduction....................................................................... Purpose: An Accessible, Sustainable and Affordable Pharmaceutical System for Alberta ......2 . Alberta Government Sponsored Drug Programs ..........................................................3 .3 Rising Costs and Sustainability Concerns .................4 .4 Perspectives of Albertans: Recent Consultation Findings ...................................5 .5 Key Components of the Alberta Pharmaceutical Strategy ...........................................6 .0 PHASE ONE: Key Components .........................................7 . Redesigning drug coverage for seniors ...................7 . Non-group drug benefit .........................................10 .3 Alignment of government drug programs ...........10 .4 Drug coverage for individuals with rare diseases ....................................................11 .5 Timely and transparent drug approval process.....12 .6 Development of a Legislative Framework .............13 3.0 PHASE TWO: Key Components ......................................4 3. Pricing and Purchasing of Drugs ............................14 3. Pharmacy Reimbursement Model ..........................15 introduction Among the key priorities . Purpose: An Accessible, Sustainable and of the Health Action Affordable Pharmaceutical System for Alberta Plan is the need to take Albertans place great value on their health system and appreciate action to develop sound the many essential services