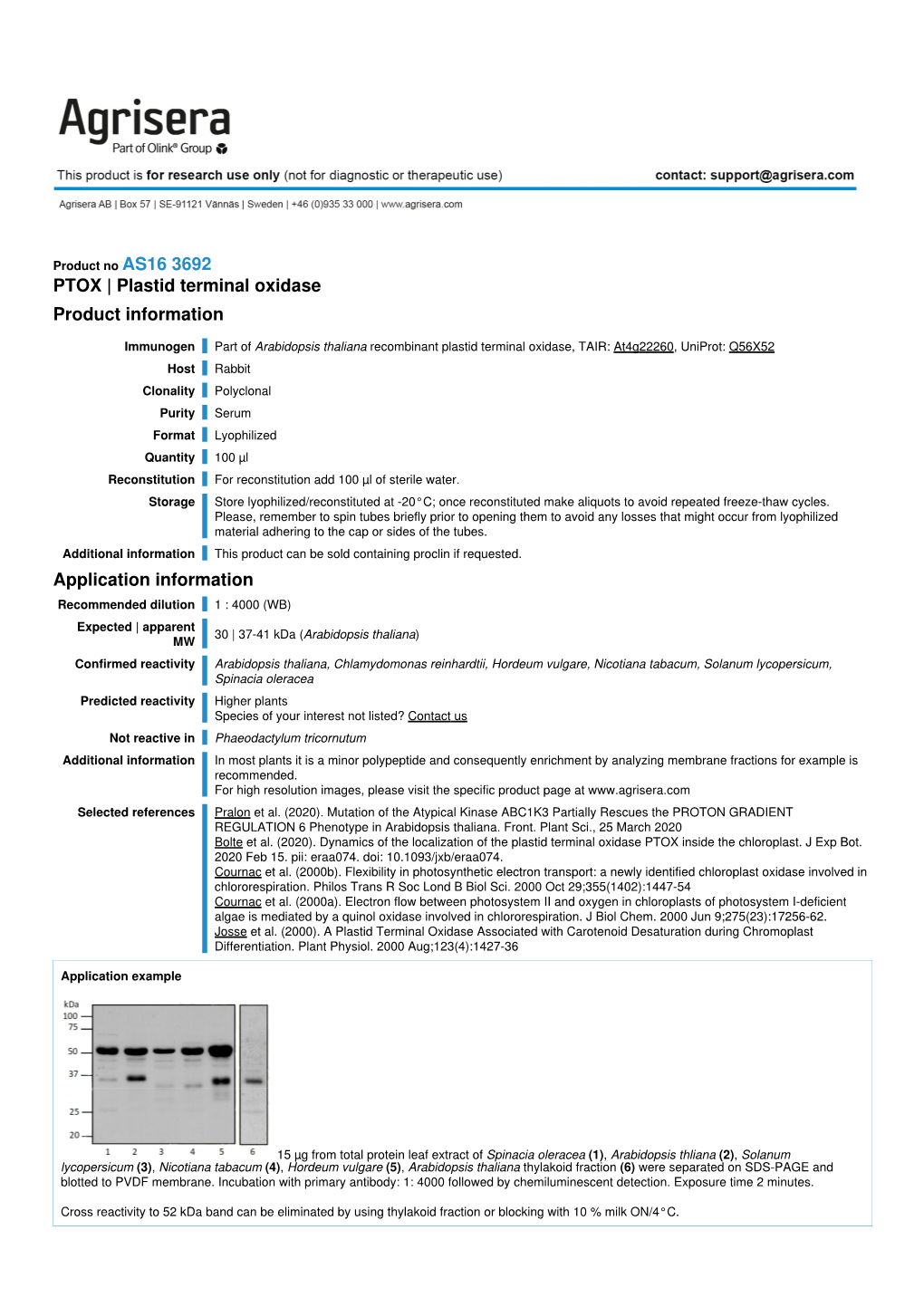
PTOX | Plastid Terminal Oxidase Product Information Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Oxidase: a Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis During Abiotic and Biotic Stress in Plants
Int. J. Mol. Sci. 2013, 14, 6805-6847; doi:10.3390/ijms14046805 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants Greg C. Vanlerberghe Department of Biological Sciences and Department of Cell and Systems Biology, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON, M1C1A4, Canada; E-Mail: [email protected]; Tel.: +1-416-208-2742; Fax: +1-416-287-7676 Received: 16 February 2013; in revised form: 8 March 2013 / Accepted: 12 March 2013 / Published: 26 March 2013 Abstract: Alternative oxidase (AOX) is a non-energy conserving terminal oxidase in the plant mitochondrial electron transport chain. While respiratory carbon oxidation pathways, electron transport, and ATP turnover are tightly coupled processes, AOX provides a means to relax this coupling, thus providing a degree of metabolic homeostasis to carbon and energy metabolism. Beside their role in primary metabolism, plant mitochondria also act as “signaling organelles”, able to influence processes such as nuclear gene expression. AOX activity can control the level of potential mitochondrial signaling molecules such as superoxide, nitric oxide and important redox couples. In this way, AOX also provides a degree of signaling homeostasis to the organelle. Evidence suggests that AOX function in metabolic and signaling homeostasis is particularly important during stress. These include abiotic stresses such as low temperature, drought, and nutrient deficiency, as well as biotic stresses such as bacterial infection. This review provides an introduction to the genetic and biochemical control of AOX respiration, as well as providing generalized examples of how AOX activity can provide metabolic and signaling homeostasis. -

Evidence for Extensive Heterotrophic Metabolism, Antioxidant Action, and Associated Regulatory Events During Winter Hardening In
Collakova et al. BMC Plant Biology 2013, 13:72 http://www.biomedcentral.com/1471-2229/13/72 RESEARCH ARTICLE Open Access Evidence for extensive heterotrophic metabolism, antioxidant action, and associated regulatory events during winter hardening in Sitka spruce Eva Collakova1, Curtis Klumas2, Haktan Suren2,3,ElijahMyers2,LenwoodSHeath4, Jason A Holliday3 and Ruth Grene1* Abstract Background: Cold acclimation in woody perennials is a metabolically intensive process, but coincides with environmental conditions that are not conducive to the generation of energy through photosynthesis. While the negative effects of low temperatures on the photosynthetic apparatus during winter have been well studied, less is known about how this is reflected at the level of gene and metabolite expression, nor how the plant generates primary metabolites needed for adaptive processes during autumn. Results: The MapMan tool revealed enrichment of the expression of genes related to mitochondrial function, antioxidant and associated regulatory activity, while changes in metabolite levels over the time course were consistent with the gene expression patterns observed. Genes related to thylakoid function were down-regulated as expected, with the exception of plastid targeted specific antioxidant gene products such as thylakoid-bound ascorbate peroxidase, components of the reactive oxygen species scavenging cycle, and the plastid terminal oxidase. In contrast, the conventional and alternative mitochondrial electron transport chains, the tricarboxylic acid cycle, and redox-associated proteins providing reactive oxygen species scavenging generated by electron transport chains functioning at low temperatures were all active. Conclusions: A regulatory mechanism linking thylakoid-bound ascorbate peroxidase action with “chloroplast dormancy” is proposed. Most importantly, the energy and substrates required for the substantial metabolic remodeling that is a hallmark of freezing acclimation could be provided by heterotrophic metabolism. -

Coversheet for Thesis in Sussex Research Online
A University of Sussex DPhil thesis Available online via Sussex Research Online: http://sro.sussex.ac.uk/ This thesis is protected by copyright which belongs to the author. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Please visit Sussex Research Online for more information and further details Structure, function and mechanism of the alternative oxidases Luke Young September 2013 Submitted in partial fulfilment towards the requirements for the degree of Doctor of Philosophy (DPhil) i | P a g e I hereby declare that this thesis has not been and will not be, submitted in whole or in part to another University for the award of any other degree. Signature ............................................. ii | P a g e University of Sussex Luke Young Submitted for the degree of Doctor of philosophy Structure function and mechanism of the alternative oxidases Summary The alternative oxidase (AOX) is the terminal protein in the alternative oxidation pathway found in plants, fungi and some protozoa. One of the more prominent protozoa that contain AOX within the bloodstream form is Trypanosoma brucei, the causative agent of human African trypanosomiasis (HAT), in which the parasite has demonstrated to be totally dependent upon the protein for continued respiration. Given the lack of AOX in mammalian cells, the protein represents an attractive chemotherapeutic target for trypanosidal activity. -

Physiological Roles of Plastid Terminal Oxidase in Plant Stress Responses
Review Physiological roles of plastid terminal oxidase in plant stress responses XIN SUN* and TAO WEN Agronomy College, Chengdu Campus, Sichuan Agricultural University, Chengdu 611130, China *Corresponding author (Email, [email protected]) The plastid terminal oxidase (PTOX) is a plastoquinol oxidase localized in the plastids of plants. It is able to transfer electrons from plastoquinone (PQ) to molecular oxygen with the formation of water. Recent studies have suggested that PTOX is beneficial for plants under environmental stresses, since it is involved in the synthesis of photoprotective carotenoids and chlororespiration, which could potentially protect the chloroplast electron transport chain (ETC) from over-reduction. The absence of PTOX in plants usually results in photo-bleached variegated leaves and impaired adaptation to environment alteration. Although PTOX level and activity has been found to increase under a wide range of stress conditions, the functions of plant PTOX in stress responses are still disputed now. In this paper, the possible physiological roles of PTOX in plant stress responses are discussed based on the recent progress. [Sun X and Wen T 2011 Physiological roles of plastid terminal oxidase in plant stress responses. J. Biosci. 36 951–956] DOI 10.1007/s12038-011- 9161-7 1. Introduction important role in chloroplast biogenesis (Carol and Kuntz 2001;Aluruet al. 2006). There was also evidence that PTOX Plastid terminal oxidase (PTOX), a plastid-localized plasto- is the terminal oxidase of chlororespiration and regulates the quinol (PQ)/O2 oxidoreductase, exists widely in photosyn- redox state of the PQ pool (Aluru and Rodermel 2004;Peltier thetic species including algae and higher plants (Carol and and Cournac 2002). -

The Plastid Terminal Oxidase: Its Elusive Function Points to Multiple Contributions to Plastid Physiology
PP66CH03-Wollman ARI 23 March 2015 13:57 The Plastid Terminal Oxidase: Its Elusive Function Points to Multiple Contributions to Plastid Physiology Wojciech J. Nawrocki,1 Nicolas J. Tourasse,2 Antoine Taly,3 Fabrice Rappaport,1 and Francis-Andre´ Wollman1,2 1Laboratoire de Physiologie Membranaire et Moleculaire´ du Chloroplaste, UMR 7141, Centre National de la Recherche Scientifique–Universite´ Pierre et Marie Curie; 2FRC 550, Centre National de la Recherche Scientifique; and 3Laboratoire de Biochimie Theorique,´ UPR 9080, Centre National de la Recherche Scientifique. Institut de Biologie Physico-Chimique, 75005 Paris, France; email: [email protected] Annu. Rev. Plant Biol. 2015. 66:49–74 Keywords First published online as a Review in Advance on PTOX, chlororespiration, chloroplast, electron transfer, structural model, January 12, 2015 carotenoid biosynthesis, redox poise, retrograde signaling The Annual Review of Plant Biology is online at plant.annualreviews.org Abstract This article’s doi: Plastids have retained from their cyanobacterial ancestor a fragment of the 10.1146/annurev-arplant-043014-114744 respiratory electron chain comprising an NADPH dehydrogenase and a di- Access provided by CNRS-Multi-Site on 06/20/15. For personal use only. Copyright c 2015 by Annual Reviews. iron oxidase, which sustain the so-called chlororespiration pathway. Despite All rights reserved Annu. Rev. Plant Biol. 2015.66:49-74. Downloaded from www.annualreviews.org its very low turnover rates compared with photosynthetic electron flow, knocking out the plastid terminal oxidase (PTOX) in plants or microalgae leads to severe phenotypes that encompass developmental and growth de- fects together with increased photosensitivity. On the basis of a phylogenetic and structural analysis of the enzyme, we discuss its physiological contribu- tion to chloroplast metabolism, with an emphasis on its critical function in setting the redox poise of the chloroplast stroma in darkness. -

Plastid Terminal Oxidase (PTOX) Has the Potential to Act As a Safety Valve for Excess Excitation Energy in the Alpine Plant Species Ranunculus Glacialis L
bs_bs_banner Plant, Cell and Environment (2013) 36, 1296–1310 doi: 10.1111/pce.12059 Plastid terminal oxidase (PTOX) has the potential to act as a safety valve for excess excitation energy in the alpine plant species Ranunculus glacialis L. CONSTANCE LAUREAU1, ROSINE DE PAEPE2, GWENDAL LATOUCHE1, MARIA MORENO-CHACÓN1, GIOVANNI FINAZZI3,4,5,6, MARCEL KUNTZ3,4,5,6, GABRIEL CORNIC1 & PETER STREB1 1Ecologie, Systématique et Evolution, Université Paris-Sud 11, UMR-CNRS 8079, Bâtiment 362, 91405 Orsay cedex, France, 2Institut de Biologie des Plantes, Université Paris-Sud 11, UMR-CNRS 8618, Bâtiment 630, 91405 Orsay cedex, France, 3Unité Mixte Recherche 5168, Laboratoire Physiologie Cellulaire et Végétale, Centre National Recherche Scientifique, F-38054 Grenoble, France, 4Commissariat à l’Energie Atomique et Energies Alternatives, l’Institut de Recherches en Technologies et Sciences pour le Vivant, F-38054 Grenoble, France, 5Université Grenoble 1, F-38041 Grenoble, France and 6Institut National Recherche Agronomique, UMR1200, F-38054 Grenoble, France ABSTRACT PSII; PFD, photon flux density; PQ, plastoquinone; PSI, pho- tosystem I; PSII, photosystem II; PTOX, plastid terminal Ranunculus glacialis leaves were tested for their plastid ter- oxidase; qL, fraction of open PSII centres; RD, dark respira- minal oxidase (PTOX) content and electron flow to pho- tion, RL, light respiration; ROS, reactive oxygen species; torespiration and to alternative acceptors. In shade-leaves, Rubisco, ribulose 1·5-bisphosphate carboxylase/oxygenase; the PTOX and NAD(P)H dehydrogenase (NDH) content SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel were markedly lower than in sun-leaves. Carbon assimilation/ electrophoresis. light and Ci response curves were not different in sun- and shade-leaves, but photosynthetic capacity was the highest in INTRODUCTION sun-leaves. -

Chlamydomonas: Molecular Genetics and Physiology Microbiology Monographs
Microbiology Monographs Series Editor: Alexander Steinbüchel Michael Hippler Editor Chlamydomonas: Molecular Genetics and Physiology Microbiology Monographs Volume 30 Series editor Alexander Steinbüchel Münster, Germany More information about this series at http://www.springer.com/series/7171 Michael Hippler Editor Chlamydomonas: Molecular Genetics and Physiology Editor Michael Hippler Institute of Plant Biology and Biotechnology Universita¨tMünster Münster, Germany ISSN 1862-5576 ISSN 1862-5584 (electronic) Microbiology Monographs ISBN 978-3-319-66363-0 ISBN 978-3-319-66365-4 (eBook) DOI 10.1007/978-3-319-66365-4 Library of Congress Control Number: 2017958132 © Springer International Publishing AG 2017 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -

Characterization of Light-Enhanced Respiration in Cyanobacteria
International Journal of Molecular Sciences Article Characterization of Light-Enhanced Respiration in Cyanobacteria Ginga Shimakawa 1,*,†,‡ , Ayaka Kohara 1,† and Chikahiro Miyake 1,2 1 Department of Biological and Environmental Science, Faculty of Agriculture, Graduate School of Agricultural Science, Kobe University, 1-1 Rokkodai, Nada, Kobe 657-8501, Japan; [email protected] (A.K.); [email protected] (C.M.) 2 Core Research for Environmental Science and Technology, Japan Science and Technology Agency, 7 Goban, Chiyoda, Tokyo 102-0076, Japan * Correspondence: [email protected]; Fax: +81-78-803-5851 † These authors contributed equally to this paper. ‡ Current address: Research Center for Solar Energy Chemistry, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8631, Japan. Abstract: In eukaryotic algae, respiratory O2 uptake is enhanced after illumination, which is called light-enhanced respiration (LER). It is likely stimulated by an increase in respiratory substrates produced during photosynthetic CO2 assimilation and function in keeping the metabolic and redox homeostasis in the light in eukaryotic cells, based on the interactions among the cytosol, chloroplasts, and mitochondria. Here, we first characterize LER in photosynthetic prokaryote cyanobacteria, in which respiration and photosynthesis share their metabolisms and electron transport chains in one cell. From the physiological analysis, the cyanobacterium Synechocystis sp. PCC 6803 performs LER, similar to eukaryotic algae, which shows a capacity comparable to the net photosynthetic O2 evolution rate. Although the respiratory and photosynthetic electron transports share the interchain, LER was uncoupled from photosynthetic electron transport. Mutant analyses demonstrated that LER is motivated by the substrates directly provided by photosynthetic CO2 assimilation, but not by glycogen. -

Flexibility in Photosynthetic Electron Transport: the Physiological Role of Plastoquinol Terminal Oxidase (PTOX)☆
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1807 (2011) 954–967 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbabio Review Flexibility in photosynthetic electron transport: The physiological role of plastoquinol terminal oxidase (PTOX)☆ Allison E. McDonald a,⁎, Alex G. Ivanov b, Rainer Bode b, Denis P. Maxwell b, Steven R. Rodermel c, Norman P.A. Hüner b a Department of Biology, Wilfrid Laurier University, Science Building, 75 University Avenue West, Waterloo, Ontario, Canada N2L 3C5 b Department of Biology and The Biotron, The University of Western Ontario, 1151 Richmond Street N., London, Ontario, Canada N6A 5B7 c Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA article info abstract Article history: Oxygenic photosynthesis depends on a highly conserved electron transport system, which must be particularly Received 15 September 2010 dynamic in its response to environmental and physiological changes, in order to avoid an excess of excitation Received in revised form 27 October 2010 energy and subsequent oxidative damage. Apart from cyclic electron flow around PSII and around PSI, several Accepted 29 October 2010 alternative electron transport pathways exist including a plastoquinol terminal oxidase (PTOX) that mediates Available online 4 November 2010 electron flow from plastoquinol to O2. The existence of PTOX was first hypothesized in 1982 and this was verified Keywords: years later based on the discovery of a non-heme, di-iron carboxylate protein localized to thylakoid membranes Immutans that displayed sequence similarity to the mitochondrial alternative oxidase. -

Alternative Oxidase: Distribution, Induction, Properties, Structure, Regulation, and Functions
ISSN 0006-2979, Biochemistry (Moscow), 2014, Vol. 79, No. 13, pp. 1615-1634. © Pleiades Publishing, Ltd., 2014. Original Russian Text © A. G. Rogov, E. I. Sukhanova, L. A. Uralskaya, D. A. Aliverdieva, R. A. Zvyagilskaya, 2014, published in Uspekhi Biologicheskoi Khimii, 2014, Vol. 54, pp. 413-456. REVIEW Alternative Oxidase: Distribution, Induction, Properties, Structure, Regulation, and Functions A. G. Rogov1*, E. I. Sukhanova1, L. A. Uralskaya1, D. A. Aliverdieva2, and R. A. Zvyagilskaya1 1Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia; E-mail: [email protected] 2Caspian Institute of Biological Resources, Dagestan Scientific Center, Russian Academy of Sciences, ul. M. Gadzhieva 45, 367025 Makhachkala, Russia; E-mail: [email protected] Received September 9, 2014 Abstract—The respiratory chain in the majority of organisms with aerobic type metabolism features the concomitant exis- tence of the phosphorylating cytochrome pathway and the cyanide- and antimycin A-insensitive oxidative route comprising a so-called alternative oxidase (AOX) as a terminal oxidase. In this review the history of AOX discovery is described. Considerable evidence is presented that AOX occurs widely in organisms at various levels of organization and is not con- fined to the plant kingdom. This enzyme has not been found only in Archaea, mammals, some yeasts and protists. Bioinformatics research revealed the sequences characteristic of AOX in representatives of various taxonomic groups. Based on multiple alignments of these sequences, a phylogenetic tree was constructed to infer their possible evolution. The ways of AOX activation, as well as regulatory interactions between AOX and the main respiratory chain are described. -

Role of Plastid Terminal Oxidase (PTOX) As a Safety Valve for Electrons
Role of Plastid Terminal Oxidase (PTOX) as a safety valve for electrons in Hordeum vulgare (Barley) plants. A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in Plant Sciences in the Faculty of Science and Engineering and 2018 Mariela P. Aguilera Miranda School of Earth and Environmental Sciences Table of Contents List of figure .................................................................................................... 7 List of tables ................................................................................................. 10 Abbreviations ................................................................................................ 11 Abstract ........................................................................................................ 14 Declaration ................................................................................................... 15 Copyright statement ...................................................................................... 16 Acknowledgements ....................................................................................... 18 Chapter 1 ......................................................................................................... 19 General Introduction ......................................................................................... 19 Introduction ................................................................................................... 20 1. Photosynthesis ..................................................................................... -

Chlamydomonas Reinhardtii†‡ Jeffrey L
EUKARYOTIC CELL, Jan. 2006, p. 26–44 Vol. 5, No. 1 1535-9778/06/$08.00ϩ0 doi:10.1128/EC.5.1.26–44.2006 Copyright © 2006, American Society for Microbiology. All Rights Reserved. Genome-Based Approaches to Understanding Phosphorus Deprivation Responses and PSR1 Control in Chlamydomonas reinhardtii†‡ Jeffrey L. Moseley,1* Chiung-Wen Chang,2 and Arthur R. Grossman1 Carnegie Institution, Department of Plant Biology, 260 Panama Street, Stanford, California 94305,1 and Department of Statistics, Sequoia Hall, 390 Serra Mall, Stanford University, Stanford, California 94305-40652 Received 4 August 2005/Accepted 31 October 2005 The Chlamydomonas reinhardtii transcription factor PSR1 is required for the control of activities involved in scavenging phosphate from the environment during periods of phosphorus limitation. Increased scavenging activity reflects the development of high-affinity phosphate transport and the expression of extracellular phosphatases that can cleave phosphate from organic compounds in the environment. A comparison of gene expression patterns using microarray analyses and quantitative PCRs with wild-type and psr1 mutant cells deprived of phosphorus has revealed that PSR1 also controls genes encoding proteins with potential “electron valve” functions—these proteins can serve as alternative electron acceptors that help prevent photodamage caused by overexcitation of the photosynthetic electron transport system. In accordance with this finding, phosphorus-starved psr1 mutants die when subjected to elevated light intensities; at these intensities, the wild-type cells still exhibit rapid growth. Acclimation to phosphorus deprivation also involves a reduction in the levels of transcripts encoding proteins involved in photosynthesis and both cytoplasmic and chloroplast translation as well as an increase in the levels of transcripts encoding stress-associated chaperones and proteases.