Fluorescence Measurements Chapter 1 - Fluorescence Theory
Total Page:16
File Type:pdf, Size:1020Kb
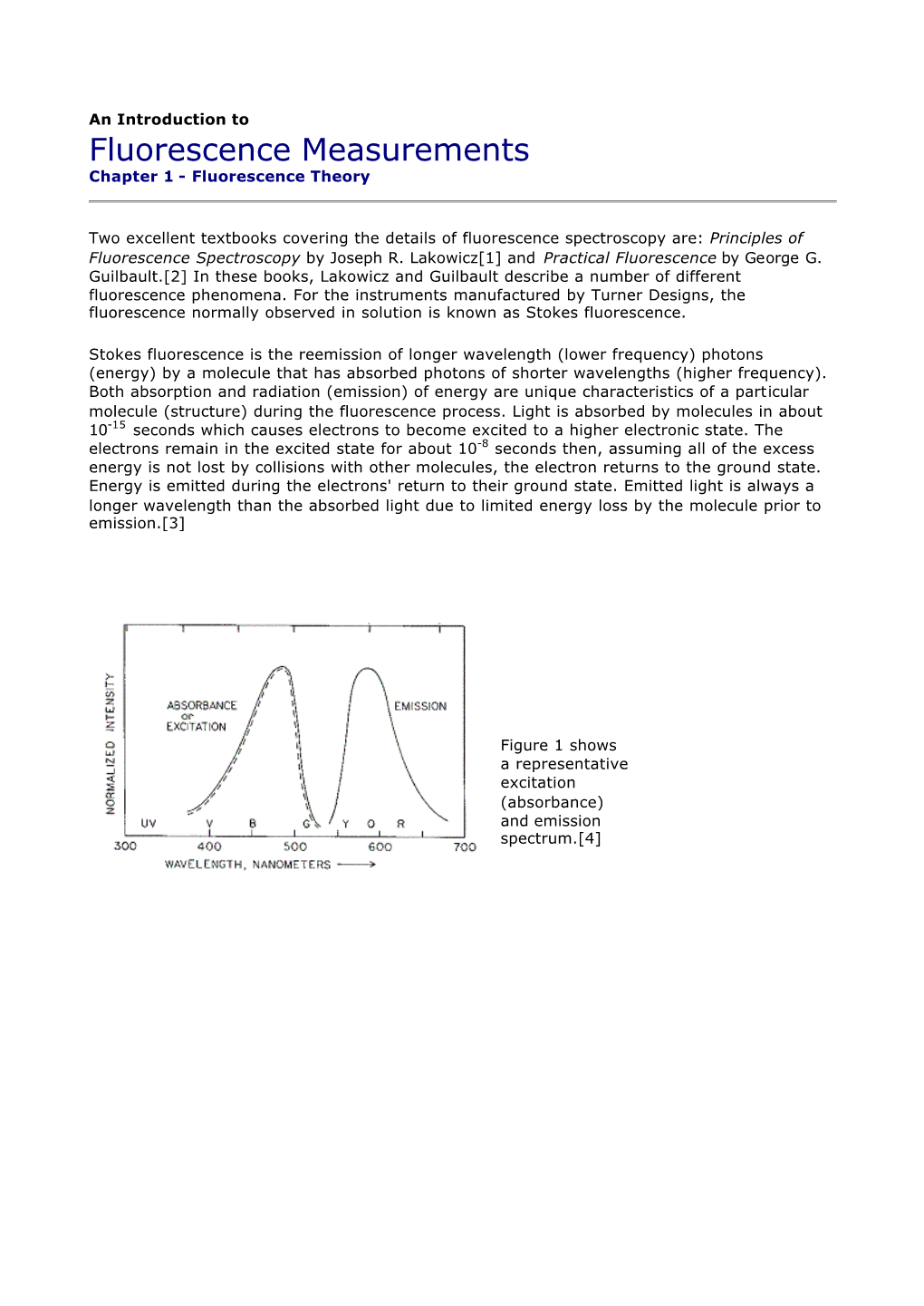
Load more
Recommended publications
-

Inexpensive, Open Source Filter Fluorometers for Measuring Relative Fluorescence Chris Stewart and John Giannini *
Inexpensive, Open Source Filter Fluorometers for Measuring Relative Fluorescence Chris Stewart and John Giannini * Biology Department, St. Olaf College, 1520 St. Olaf Avenue, Northfield, MN 55057 5 ABSTRACT Expanding upon the work of other teams, we provide two different designs for making inexpensive, single beam, filter fluorometers that can be used to measure the relative fluorescence of different chemicals in solution and demonstrate basic principles of fluorometry. Like other instruments that we have developed, the first model can be 10 assembled from 3D-printed parts, and the second version can be built using supplies available at most hardware stores or online. Both models use: (i) a sensitive light dependent resistor (LDR) connected to a digital multimeter as their detector; (ii) a tactical LED flashlight with a convex lens and adjustable head to focus the beam as the light source; and (iii) pieces of colored cellophane as their excitation and emission 15 filters. In addition, both fluorometers contain a 4x objective lens from a compound microscope to further focus the light beam and increase its intensity. We tested these models using increasing concentrations of two common fluorophores (Rhodamine B and Acridine Orange) in solution, and we found that the instruments generated data and trends similar to those of other devices described in the literature. We further explain 20 how these fluorometers can be used in a chemistry, biochemistry, biology, or physics course to illustrate some of the basic principles of fluorometry, such as how these instruments are designed and built and how the intensity of a fluorophore in solution varies with its concentration. -

Methods for Strand-Specific DNA Detection with Cationic Conjugated Polymers Suitable for Incorporation Into DNA Chips and Microarrays
Methods for strand-specific DNA detection with cationic conjugated polymers suitable for incorporation into DNA chips and microarrays Bin Liu† and Guillermo C. Bazan† Departments of Chemistry and Biochemistry and Materials, Institute for Polymers and Organic Solids, University of California, Santa Barbara, CA 93106 Communicated by Alan J. Heeger, University of California, Santa Barbara, CA, November 17, 2004 (received for review October 11, 2004) A strand-specific DNA sensory method is described based on green) to the PNA sequence. The PNA-C* and the ssDNA are surface-bound peptide nucleic acids and water-soluble cationic first treated according to hybridization protocols, and the CCP conjugated polymers. The main transduction mechanism operates is added to the resulting solution. If the ssDNA does not by taking advantage of the net increase in negative charge at hybridize, one encounters situation A in Scheme 1, where the the peptide nucleic acid surface that occurs upon single-stranded ssDNA and the CCP are brought together by nonspecific elec- DNA hybridization. Electrostatic forces cause the oppositely trostatic forces. The PNA-C* is not incorporated into the charged cationic conjugated polymer to bind selectively to the ‘‘com- electrostatic complex. Situation B in Scheme 1 shows that when plementary’’ surfaces. This approach circumvents the current need the PNA-C* and the complementary ssDNA hybridize, the CCP to label the probe or target strands. The polymer used in these binds to the duplex structure. If the optical properties of the assays is poly[9,9-bis(6-N,N,N-trimethylammonium)hexyl)fluorene- CCP and the C* are optimized, then selective excitation of the co-alt-4,7-(2,1,3-benzothiadiazole) dibromide], which was specifi- CCP results in very efficient FRET to C*. -

Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells
14234 • The Journal of Neuroscience, October 21, 2015 • 35(42):14234–14250 Neurobiology of Disease Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells Marija Usenovic, Shahriar Niroomand, Robert E. Drolet, Lihang Yao, Renee C. Gaspar, Nathan G. Hatcher, Joel Schachter, John J. Renger, and Sophie Parmentier-Batteur Neuroscience, Merck Research Laboratories, West Point, Pennsylvania 19486 Neuronal inclusions of hyperphosphorylated and aggregated tau protein are a pathological hallmark of several neurodegenerative tauopathies, including Alzheimer’s disease (AD). The hypothesis of tau transmission in AD has emerged from histopathological studies of the spatial and temporal progression of tau pathology in postmortem patient brains. Increasing evidence in cellular and animal models supports the phenomenon of intercellular spreading of tau. However, the molecular and cellular mechanisms of pathogenic tau trans- mission remain unknown. The studies described herein investigate tau pathology propagation using human neurons derived from induced pluripotent stem cells. Neurons were seeded with full-length human tau monomers and oligomers and chronic effects on neuronal viability and function were examined over time. Tau oligomer-treated neurons exhibited an increase in aggregated and phos- phorylated pathological tau. These effects were associated with neurite retraction, loss of synapses, aberrant calcium homeostasis, and imbalanced neurotransmitter release. In contrast, tau monomer treatment did not produce any measureable changes. This work sup- ports the hypothesis that tau oligomers are toxic species that can drive the spread of tau pathology and neurodegeneration. Key words: Alzheimer’s disease; hiPSC neurons; neurodegeneration; pathology propagation; tau oligomer seeds Significance Statement Several independent studies have implicated tau protein as central to Alzheimer’s disease progression and cell-to-cell pathology propagation. -

Fluorescence Spectrophotometry Is a Class of Techniques That Assay the State of a Biological Instrumentation for Fluorescence Spectrophotometry
Fluorescence Introductory article Spectrophotometry Article Contents . The Electronic Excited State Peter TC So, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA . Radiative and Nonradiative Decay Pathways Chen Y Dong, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA . Factors Affecting Fluorescence Intensity . Phosphorescence . Fluorescence spectrophotometry is a class of techniques that assay the state of a biological Instrumentation for Fluorescence Spectrophotometry . Applications of Fluorescence in the Study of Biological system by studying its interactions with fluorescent probe molecules. This interaction is Structure and Function monitored by measuring the changes in the fluorescent probe optical properties. The Electronic Excited State indistinguishability of electrons and the Pauli exclusion Fluorescence and phosphorescence are photon emission principle require the electronic wave functions to have processes that occur during molecular relaxation from either symmetric or asymmetric spin states. The symmetric electronic excited states. These photonic processes involve wave functions, also called the triple state, have three transitions between electronic and vibrational states of forms, multiplicity of three. The antisymmetric wave polyatomic fluorescent molecules (fluorophores). The function, also called the singlet state, has one form, Jablonski diagram (Figure 1) offers a convenient represen- multiplicity of one. tation of the excited state structure and the relevant To the first order, optical transition couples states with transitions. Electronic states are typically separated by the same multiplicity. Optical transition excites the energies on the order of 10 000 cm 2 1. Each electronic state molecules from the lowest vibrational level of the electronic is split into multiple sublevels representing the vibrational ground state to an accessible vibrational level in an ele- modes of the molecule. -

Development of Potential Strategies for DNA Quantification and DNA Probes for Detection of Legionella Pneumophila
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2019 Development of potential strategies for DNA quantification and DNA probes for detection of Legionella pneumophila Parker James Dulin Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Civil Engineering Commons, and the Environmental Engineering Commons Recommended Citation Dulin, Parker James, "Development of potential strategies for DNA quantification and DNA probes for detection of Legionella pneumophila" (2019). Graduate Theses and Dissertations. 17673. https://lib.dr.iastate.edu/etd/17673 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Development of potential strategies for DNA quantification and DNA probes for detection of Legionella pneumophila by Parker James Dulin A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Civil Engineering (Environmental Engineering) Program of Study Committee: Kaoru Ikuma, Co-major Professor Carmen Gomes, Co-major Professor Chris Rehmann The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2019 Copyright © Parker James Dulin, 2019. -
Recommendations and Guidelines for Standardization of Fluorescence Spectroscopy
NISTIR 7457 Recommendations and Guidelines for Standardization of Fluorescence Spectroscopy Paul C. DeRose Biochemical Science Division National Institute of Standards and Technology Gaithersburg, MD 20899-8312 1 NISTIR 7457 Recommendations and Guidelines for Standardization of Fluorescence Spectroscopy Paul C. DeRose Biochemical Science Division National Institute of Standards and Technology Gaithersburg, MD 20899-8312 October 2007 U.S. DEPARTMENT OF COMMERCE Carlos M. Gutierrez,Secretary TECHNOLOGY ADMINISTRATION Robert C. Cresanti, Under Secretary of Commerce for Technology NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY James M. Turner, Acting Director 2 Table of Contents 1. INTRODUCTION ................................................................................... 4 2. QUALITATIVE AND QUANTITATIVE FLUORESCENCE MEASUREMENTS........................................................................................ 6 2.1 Qualitative Fluorescence Measurements ............................................................ 6 2.2 Quantitative Fluorescence Measurements .......................................................... 7 3. FACTORS AFFECTING QUANTIFICATION ..................................... 7 3.1 Instrument-Based Factors ................................................................................... 7 3.2 Sample-Based Factors......................................................................................... 8 4. APPARATUS COMPONENTS.............................................................. 9 4.1 Excitation Light Source -

Fluorolog®-3 with Fluoressence™
Fluorolog-3 Operation Manual rev. G (2 May 2014) Fluorolog®-3 with FluorEssence™ Operation Manual www.HORIBA.com/scientific rev. G i Fluorolog-3 Operation Manual rev. G (2 May 2014) Copyright © 2002–2014 by HORIBA Instruments Incorporated. All rights reserved. No part of this work may be reproduced, stored, in a retrieval system, or transmitted in any form by any means, including electronic or mechanical, photocopying and recording, without prior written permission from HORIBA Instruments Incorporated. Requests for permission should be requested in writing. Origin® is a registered trademark of OriginLab Corporation. Alconox® is a registered trademark of Alconox, Inc. Ludox® is a registered trademark of W.R. Grace and Co. Teflon® is a registered trademark of E.I. du Pont de Nemours and Company. Windows® and Excel® are registered trademarks of Microsoft Corporation. Information in this manual is subject to change without notice, and does not represent a commitment on the part of the vendor. May 2014 Part number J81014 ii Fluorolog-3 Operation Manual rev. G (2 May 2014) Table of Contents 0: Introduction ................................................................................................. 0-1 About the Fluorolog®-3 ........................................................................................................................... 0-1 Chapter overview .................................................................................................................................... 0-2 Disclaimer .............................................................................................................................................. -

Fluorescence Workshop UMN Physics June 8-10, 2006 Steady-State Fluorometer Joachim Mueller
Fluorescence Workshop UMN Physics June 8-10, 2006 Steady-state Fluorometer Joachim Mueller Instrumental Setup, light sources, wavelength selection, detectors, polarizers, corrections, inner filter effect ISS PC1 (ISS Inc., Champaign, IL, USA) Fluorolog-3 (Jobin Yvon Inc, Edison, NJ, USA ) QuantaMaster (OBB Sales, London, Ontario N6E 2S8) Fluorometer Components Excitation Polarizer Sample Emission Excitation Wavelength Light Source Polarizer Selection Emission Wavelength Computer Selection Fluorometer: DetectorThe Basics Note: Both polarizers can be removed from the optical beam path OPTICAL LAYOUT: PTI Model QM-4 1 Lamp housing 2 Adjustable slits 3 Excitation Monochromator 4 Sample compartment 5 Baffle 6 Filter holders 7 Excitation/emission optics 8 Cuvette holder 9 Emission port shutter 10 Excitation Correction 11 Emission Monochromator 12 PMT detector Light Sources Lamp Light Sources Xenon Arc Lamp Profiles 1. Xenon Arc Lamp (wide Ozone Free range of wavelengths) Visible UV 2. High Pressure Mercury Lamps (High Intensities but concentrated in specific lines) Mercury-Xenon Arc Lamp Profile 3. Mercury-Xenon Arc Lamp (greater intensities in the UV) 4. Tungsten-Halogen Lamps Detectors Photomultiplier tube (PMT) http://micro.magnet.fsu.edu/primer/flash/photomultiplier Principle of operation: Pictures: Photon Counting (Digital) and Analog Detection time Continuous Current Measurement Signal Photon Counting: Analog: Constant Variable High Voltage Supply Voltage Supply PMT PMT level Anode Current Discriminator = Sets Level TTL Output Pulse averaging -

Standard Guide to Fluorescence Instrument Calibration and Correction
NISTIR 7458 Standard Guide to Fluorescence - Instrument Calibration and Validation Paul C. DeRose Biochemical Science Division National Institute of Standards and Technology Gaithersburg, MD 20899-8312 1 NISTIR 7458 Standard Guide to Fluorescence - Instrument Calibration and Validation Paul C. DeRose Biochemical Science Division National Institute of Standards and Technology Gaithersburg, MD 20899-8312 October 2007 U.S. DEPARTMENT OF COMMERCE Carlos M. Gutierrez,Secretary TECHNOLOGY ADMINISTRATION Robert C. Cresanti,Under Secretary of Commerce for Technology NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY James M. Turner, Acting Director 2 Table of Contents 1. Scope........................................................................................................ 4 2. Wavelength Accuracy.............................................................................. 4 2.1 Low Pressure Atomic Lamps.............................................................................. 5 2.2 Dy-YAG crystal.................................................................................................. 5 2.3 Eu-doped glass or PMMA .................................................................................. 5 2.4 Anthracene-doped PMMA ................................................................................. 7 2.5 Ho2O3 solution or doped glass with diffuse reflector, scatterer or fluorescent dye ............................................................................................................................ 7 2.6 Xe Source -

Fluorescence Spectroscopy
TECHNICAL NOTE Fluorescence Spectroscopy Yevgen Povrozin and Beniamino Barbieri Published in Handbook of Measurement in Science and Engineering, vol. 3; Myer Kurtz, editor, John Wiley & Sons, 2016. (Published on the ISS web site with permission of the Editor) Table of Contents 1. Observables measured in fluorescence ..................................................................... 2475 2. The Perrin-Jablońsky diagram .................................................................................... 2476 3. Instrumentation ............................................................................................................ 2479 5.1 Light Source ........................................................................................................................ 2479 5.2 Monochromator ................................................................................................................... 2480 5.3 Light Detectors .................................................................................................................... 2481 5.4 Instrumentation for steady-state fluorescence: analog and photon counting ..................... 2483 5.5 The Measurement of decay times: Frequency-domain and time-domain techniques ........ 2483 6 Fluorophores ................................................................................................................ 2486 7 Measurements .............................................................................................................. 2486 7.1 Excitation spectrum ............................................................................................................ -

Fluorescence and Fluorescence Applications
Fluorescence and Fluorescence Applications Contents 1. Introduction ................................................................................................................................ 1 2. Principles of Fluorescence .......................................................................................................... 1 3. Fluorescence Spectra .................................................................................................................. 3 4. Fluorescence Resonance Energy Transfer (FRET) ....................................................................... 5 5. Dark Quenchers .......................................................................................................................... 7 6. Proximal G-base Quenching ........................................................................................................ 8 7. Intercalating Dyes ..................................................................................................................... 10 8. Dual-labeled Probes .................................................................................................................. 10 8.1 Hydrolysis Probes for the 5’ Nuclease Assay ...................................................................... 10 8.2 Molecular Beacons .............................................................................................................. 11 8.3 Hybridization/FRET probes ................................................................................................. 11 8.4 ScorpionsTM -

DNA Forensics Using Single Molecule Technology
The author(s) shown below used Federal funding provided by the U.S. Department of Justice to prepare the following resource: Document Title: DNA Forensics Using Single Molecule Technology: From DNA Recovery and Extraction to Genotyping Degraded and Trace Evidence without PCR Author(s): Matthew Antonik Document Number: 251812 Date Received: July 2018 Award Number: 2011-DN-BX-K542 This resource has not been published by the U.S. Department of Justice. This resource is being made publically available through the Office of Justice Programs’ National Criminal Justice Reference Service. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Final Technical Report Title: DNA forensics using single molecule technology: from DNA recovery and extraction to genotyping degraded and trace evidence without PCR Award: 2011-DN-BX-K542 Author: Matthew Antonik Abstract PCR based DNA profiling technology is the gold standard for human identification technology, being very discriminatory and requiring only several cells worth of DNA. However it is still useful to consider alternative technologies which fundamentally re-imagine how forensic DNA evidence is characterized and typed. Wholly new approaches may provide supplemental information under particularly challenging conditions, for example with low copy numbers samples, mixtures, and damaged DNA (eg. abasic sites or fragmented loci). Alternatively, novel approaches may be developed that don't require the time or training associated with current typing methods, making DNA profiling more economical and broadly available. One concept is to apply contemporary single molecule techniques to forensic analysis, with the goal of being able to interrogate DNA samples one molecule at a time, measuring each molecule repeatedly to guarantee confidence in the result.