ΧΏΡΑ ΤΜΉΜΑ Ιαπωνία Plants Or Establishments Manufacturing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp
Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 1332 Nippon Suisan Kaisha, Ltd. 50 1333 Maruha Nichiro Corp. 500 1605 Inpex Corp. 125 1721 Comsys Holdings Corp. 50 1801 Taisei Corp. 50 1802 Obayashi Corp. 50 1803 Shimizu Corp. 50 1808 Haseko Corp. 250 1812 Kajima Corp. 50 1925 Daiwa House Industry Co., Ltd. 50 1928 Sekisui House, Ltd. 50 1963 JGC Corp. 50 2002 Nisshin Seifun Group Inc. 50 2269 Meiji Holdings Co., Ltd. 250 2282 Nh Foods Ltd. 50 2432 DeNA Co., Ltd. 500/3 2501 Sapporo Holdings Ltd. 250 2502 Asahi Group Holdings, Ltd. 50 2503 Kirin Holdings Co., Ltd. 50 2531 Takara Holdings Inc. 50 2768 Sojitz Corp. 500 2801 Kikkoman Corp. 50 2802 Ajinomoto Co., Inc. 50 2871 Nichirei Corp. 100 2914 Japan Tobacco Inc. 50 3086 J.Front Retailing Co., Ltd. 100 3099 Isetan Mitsukoshi Holdings Ltd. 50 3101 Toyobo Co., Ltd. 50 3103 Unitika Ltd. 50 3105 Nisshinbo Holdings Inc. 50 3289 Tokyu Fudosan Holdings Corp. 50 3382 Seven & i Holdings Co., Ltd. 50 3401 Teijin Ltd. 250 3402 Toray Industries, Inc. 50 3405 Kuraray Co., Ltd. 50 3407 Asahi Kasei Corp. 50 3436 SUMCO Corp. 500 3861 Oji Holdings Corp. 50 3863 Nippon Paper Industries Co., Ltd. 500 3865 Hokuetsu Kishu Paper Co., Ltd. 50 4004 Showa Denko K.K. 500 4005 Sumitomo Chemical Co., Ltd. 50 4021 Nissan Chemical Industries, Ltd. 50 4042 Tosoh Corp. 50 4043 Tokuyama Corp. 50 WF-101-E-20170803 Copyright © Nikkei Inc. All rights reserved. 1/5 Nikkei Stock Average - Par Value (Update:August/1, 2017) Code Company Name Par Value(Yen) 4061 Denka Co., Ltd. -

Download the Full GTC ONE Minute Brief
Equity | Currencies & Commodities | Corporate & Global Economic News | Technical Snapshot | Economic Calendar 7 August 2018 Economic and political news Key indices Eskom workers have been given a deadline until tomorrow to either 1 Day 1 D % WTD % MTD % Prev. month YTD % accept or reject a new wage offer that includes one-off cash payments of Last close ZAR10,000. Chg Chg Chg Chg % Chg Chg JSE All Share 56861.21 -256.82 -0.45 -0.45 -0.99 -0.31 -4.44 The Democratic Alliance has given the SABC an ultimatum to air a video of party leader, Mmusi Maimane, expressing the party’s position on land JSE Top 40 50784.44 -204.02 -0.40 -0.40 -1.03 -0.39 -3.33 reform. FTSE 100 7663.78 4.68 0.06 0.06 -1.10 1.46 -0.31 Cape Town Mayor, Patricia de Lille, stated that she would continue pursuing legal action against at least 12 individuals who defamed her DAX 30 12598.21 -17.55 -0.14 -0.14 -1.62 4.06 -2.47 over the last six months. CAC 40 5477.18 -1.80 -0.03 -0.03 -0.62 3.53 3.10 South Africa’s (SA) largest organised farmers' body, Agri SA, will keep an S&P 500 2850.40 10.05 0.35 0.35 1.21 3.60 6.61 eye on the African National Congress' (ANC) plans to target 139 farms in Nasdaq test cases for expropriation without compensation and would go to the 7859.68 47.66 0.61 0.61 2.45 2.15 13.85 Constitutional Court if necessary. -

1332:Xtks Nippon Suisan Kaisha Ltd 3 4 1 1334:Xtks Maruha Nichiro Holdings Inc. 3 4 1 1377:Xtks Sakata Seed Corp. 3 5 2 1414:Xtks SHO-BOND Holdings Co
Symbol Code Description Current Rating New rating Diff 1332:xtks Nippon Suisan Kaisha Ltd 3 4 1 1334:xtks Maruha Nichiro Holdings Inc. 3 4 1 1377:xtks Sakata Seed Corp. 3 5 2 1414:xtks SHO-BOND Holdings Co. Ltd 3 6 3 1766:xtks TOKEN Corp. 3 6 3 1801:xtks Taisei Corp. 3 5 2 1803:xtks Shimizu Corp. 3 4 1 1808:xtks Haseko Corp. 3 4 1 1812:xtks Kajima Corp. 3 5 2 1820:xtks Nishimatsu Construction Co. Ltd 3 6 3 1824:xtks Maeda Corp. 3 6 3 1833:xtks Okumura Corp. 3 6 3 1860:xtks Toda Corp. 3 5 2 1861:xtks Kumagai Gumi Co. Ltd 3 8 5 1865:xtks Asunaro Aoki Construction Co. Ltd 3 6 3 1870:xtks Yahagi Construction Co. Ltd 3 4 1 1881:xtks NIPPO Corp. 3 6 3 1883:xtks Maeda Road Construction Co. Ltd 3 6 3 1911:xtks Sumitomo Forestry Co Ltd 3 4 1 1924:xtks PanaHome Corp. 3 4 1 1925:xtks Daiwa House Industry Co. Ltd 3 4 1 1928:xtks Sekisui House Ltd 3 4 1 1934:xtks YURTEC Corp. 3 6 3 1945:xtks Tokyo Energy & Systems Inc. 3 4 1 1961:xtks Sanki Engineering Co. Ltd 3 4 1 1963:xtks JGC Corporation 3 4 1 1968:xtks Taihei Dengyo Kaisha Ltd 3 4 1 1969:xtks Takasago Thermal Engineering Co. Ltd 3 4 1 1973:xtks NEC Networks & System Integration Corp. 3 5 2 1979:xtks Taikisha Ltd 3 4 1 1983:xtks TOSHIBA PLANT SYSTEMS & SERVICES Corp. -

Introduction
Hypertension Research (2014) 37, 256–259 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr GUIDELINES (JSH 2014) Introduction Hypertension Research (2014) 37, 256–259; doi:10.1038/hr.2014.18 The Japanese Society of Hypertension revised the Japanese Society of JSH 2014 should also be used by health nurses, nurses, dietitians and Hypertension Guidelines for the Management of Hypertension in staff responsible for team practice for hypertension management. 2009 (JSH 2009) and published the JSH 2014. Basically, the JSH 2014 Therefore, in addition to specialists in hypertension, the members of was prepared according to strategies to prepare the JSH 2009 and the The Japan Association of Medical Practitioners, The Japanese Society ‘Guidance for the Preparation of Treatment Guidelines in 2007’ of Clinical Pharmacology and Therapeutics, Japan Pharmaceutical established by the Medical Information Network Distribution Service. Association, Japanese Society of Clinical Nutrition, and Patient In the ‘Introduction’ section, methods to prepare the JSH 2014 are Corporation belong to the Japanese Society of Hypertension Com- introduced. mittee for Guidelines for the Management of Hypertension. With regard to the affiliations of the committee members, their occupation, 1. OBJECTIVE AND SUBJECTS OF THE JSH 2014 affiliated corporations and positions are described. Hypertension causes stroke (cerebral infarction, cerebral hemorrhage, subarachnoidal hemorrhage), heart disease (coronary artery disease, 2. COMPOSITION OF THE JAPANESE SOCIETY OF cardiac hypertrophy, heart failure), kidney disease (nephrosclerosis) HYPERTENSION COMMITTEE FOR GUIDELINES FOR THE and macrovascular disease. Therefore, the primary objective MANAGEMENT OF HYPERTENSION of the JSH 2014 is to present standard treatment to prevent the The Japanese Society of Hypertension Guidelines for the Management onset/progression of hypertensive complications of the brain/heart/ of Hypertension is official. -
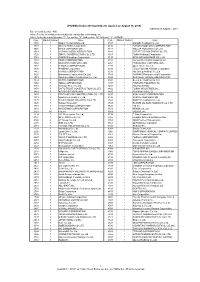
"JPX-Nikkei Index 400"
JPX-Nikkei Index 400 Constituents (applied on August 30, 2019) Published on August 7, 2019 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3107 1 Daiwabo Holdings Co.,Ltd. 1333 1 Maruha Nichiro Corporation 3116 1 TOYOTA BOSHOKU CORPORATION 1605 1 INPEX CORPORATION 3141 1 WELCIA HOLDINGS CO.,LTD. 1719 1 HAZAMA ANDO CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3167 1 TOKAI Holdings Corporation 1721 1 COMSYS Holdings Corporation 3197 1 SKYLARK HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1802 1 OBAYASHI CORPORATION 3254 1 PRESSANCE CORPORATION 1803 1 SHIMIZU CORPORATION 3288 1 Open House Co.,Ltd. 1808 1 HASEKO Corporation 3289 1 Tokyu Fudosan Holdings Corporation 1812 1 KAJIMA CORPORATION 3291 1 Iida Group Holdings Co.,Ltd. 1820 1 Nishimatsu Construction Co.,Ltd. 3349 1 COSMOS Pharmaceutical Corporation 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1824 1 MAEDA CORPORATION 3382 1 Seven & I Holdings Co.,Ltd. 1860 1 TODA CORPORATION 3391 1 TSURUHA HOLDINGS INC. 1861 1 Kumagai Gumi Co.,Ltd. 3401 1 TEIJIN LIMITED 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3402 1 TORAY INDUSTRIES,INC. 1881 1 NIPPO CORPORATION 3405 1 KURARAY CO.,LTD. 1893 1 PENTA-OCEAN CONSTRUCTION CO.,LTD. 3407 1 ASAHI KASEI CORPORATION 1911 1 Sumitomo Forestry Co.,Ltd. -

Perfect Storm Profits at Risk in the Japanese Seafood Industry
PERFECT STORM PROFITS AT RISK IN THE JAPANESE SEAFOOD INDUSTRY SEPTEMBER 2019 FUNDERS Planet Tracker [email protected] DISCLAIMER Investor Watch’s reports are impersonal and do not provide individualized advice or recommendations for any specific reader or portfolio. Investor Watch is not an investment adviser and makes no recommendations regarding the advisability of investing in any particular company, investment fund or other vehicle. The information contained in this research report does not constitute an offer to sell securities or the solicitation of an offer to buy, or recommendation for investment in, any securities within any jurisdiction. The information is not intended as financial advice. The information used to compile this report has been collected from a number of sources in the public domain and from Investor Watch licensors. While Investor Watch and its partners have obtained information believed to be reliable, none of them shall be liable for any claims or losses of any nature in connection with information contained in this document, including but not limited to, lost profits or punitive or consequential damages. This research report provides general information only. The information and opinions constitute a judgment as at the date indicated and are subject to change without notice. The information may therefore not be accurate or current. The information and opinions contained in this report have been compiled or arrived at from sources believed to be reliable and in good faith, but no representation or warranty, express or implied, is made by Investor Watch as to their accuracy, completeness or correctness and Investor Watch does also not warrant that the information is up-to-date. -

Annual Report 2016 for the Year Ended December 31, 2016
Annual Report 2016 For the year ended December 31, 2016 Leaping Forward Contents Review of Operations Editorial Policy 02 (Contents/Editorial Policy) 18 (Pharmaceuticals Business/Bio-chemicals Business) This is our Annual Report for 2016. The environment surrounding the pharmaceuticals industry is rapidly changing, and there Our Philosophy Corporate Governance is a movement to dramatically revise the framework that has (Management Philosophy/Core Values) (Board of Directors/Corporate Governance) 03 31 underpinned the industry thus far. Despite this environment, “human resources” and “technology” will continue to be the Who we are Compliance baseline for innovation at Kyowa Hakko Kirin Group, which 04 (Group Structure/Business Model) 35 (Compliance/Risk Management) aspires to realize health and well-being. In the special features, we describe our human resource development that is promoting our globalization and our production technology for biopharmaceuticals. Niro Sakamoto, Executive Officer, Director of What we do Dialogue Please deepen your understanding of our group, which is taking Corporate Communications 06 (FY2016-2020 Mid-term Business Plan) 38 (Vice President × Outside Directors) initiatives to make the leap forward to become a Global Specialty Department Pharmaceutical Company (GSP). High Top Message Financial Information 07 41 Importance to Stakeholders Annual Report (PDF version) http://ir.kyowa-kirin.com/en/library/annual_report.html Special Feature Kyowa Hakko Kirin Website Special Feature 1: Human Resource Development Investor -

Pasetocin and Sawacillin
Pasetocin® and Sawacillin®: Joint Application Seeking Approval for Additional Indication for Helicobacter pylori Eradication by Triple Therapy with Proton Pump Inhibitors and either Clarithromycin or Metronidazole Tokyo, August 31, 2012 - Kyowa Hakko Kirin Co., Ltd. (“Kyowa Hakko Kirin”; Tokyo:4151; President and CEO: Nobuo Hanai) and Astellas Pharma Inc. (“Astellas Pharma”; Tokyo:4503; President and CEO: Yoshihiko Hatanaka) announced today that they have submitted a joint application* to Japan’s Ministry of Health, Labour and Welfare seeking approval of Helicobacter pylori (“H. pylori”) gastritis as an additional indication for H. pylori eradication by triple therapy including amoxicillin hydrate (generic name; brand names: “Pasetocin® Capsules 125 and 250; Pasetocin® Tablets 250”, “Sawacillin® Capsules 125 and 250; Sawacillin® Tablets 250” and one other brand). This concomitant therapy consists of a proton pump inhibitor (lansoprazole, omeprazole, rabeprazole sodium and esomeprazole magnesium hydrate, generic name; marketed under five brand names), amoxicillin hydrate, and either clarithromycin (generic name; marketed under two brand names) or metronidazole (generic name; marketed under one brand name). H. pylori gastritis is also known as chronic active gastritis due to the fact that it causes histological gastric mucosal injury as a result of the persistent infiltration of inflammatory cells in the gastric mucosa due to H. pylori infection, and is believed to be associated with the development of various H. pylori-related diseases such as gastric and duodenal ulcers. However, under the Japanese National Health Insurance (NHI) system, the approved indications for eradication of H. pylori are currently limited to gastric and duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, and the stomach after endoscopic resection of early stage gastric cancer. -

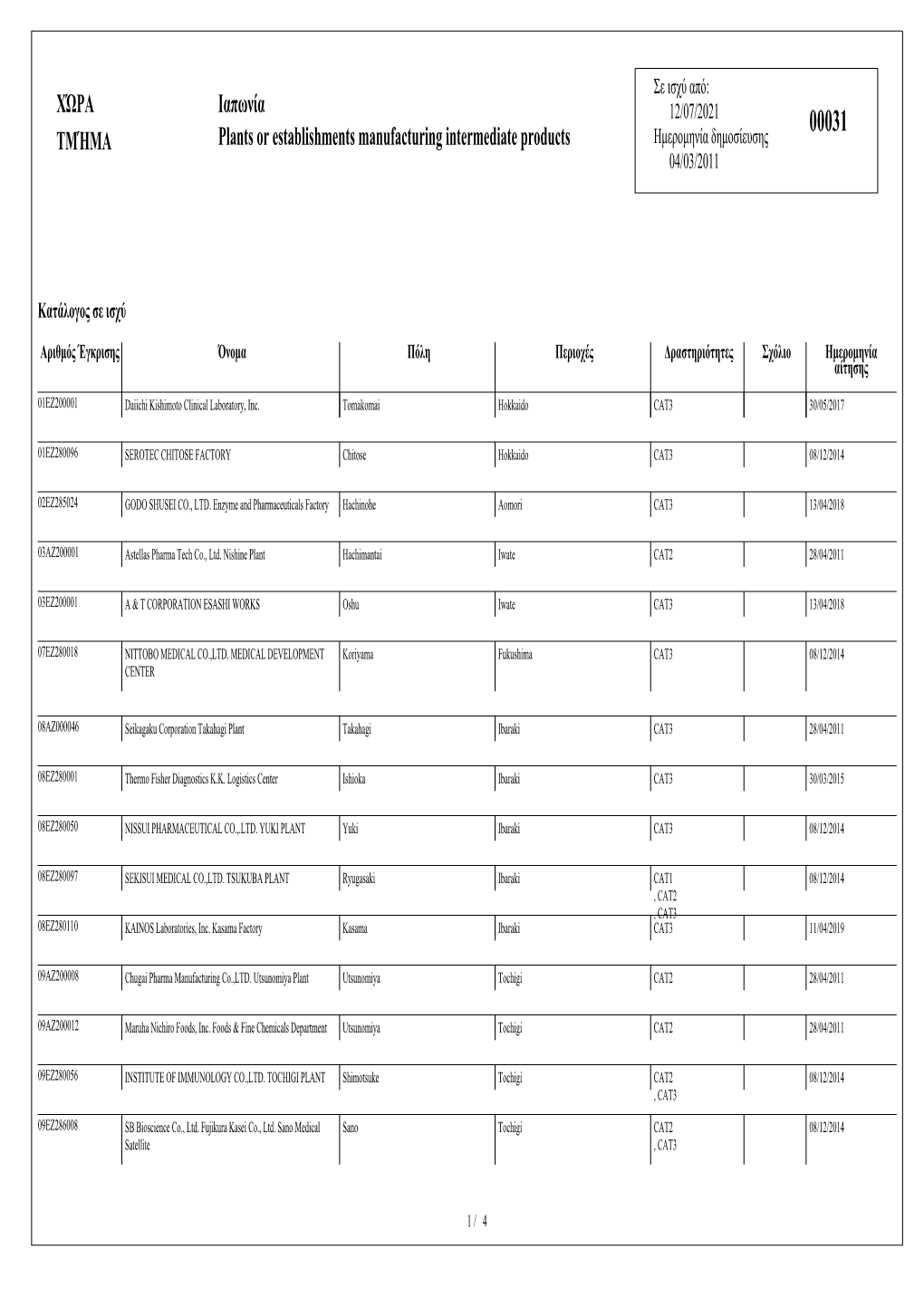
PAYS SECTION Japon Manufactures De Produits Intermédiaires
En vigueur depuis le: PAYS Japon 12/07/2021 00031 SECTION Manufactures de produits intermédiaires Date de publication 04/03/2011 Liste en vigueur Numéro Nom Ville Régions Activités Remarque Date de la demande d'agrément 01EZ200001 Daiichi Kishimoto Clinical Laboratory, Inc. Tomakomai Hokkaido CAT3 30/05/2017 01EZ280096 SEROTEC CHITOSE FACTORY Chitose Hokkaido CAT3 08/12/2014 02EZ285024 GODO SHUSEI CO., LTD. Enzyme and Pharmaceuticals Factory Hachinohe Aomori CAT3 13/04/2018 03AZ200001 Astellas Pharma Tech Co., Ltd. Nishine Plant Hachimantai Iwate CAT 2 28/04/2011 03EZ200001 A & T CORPORATION ESASHI WORKS Oshu Iwate CAT3 13/04/2018 07EZ280018 NITTOBO MEDICAL CO.,LTD. MEDICAL DEVELOPMENT Koriyama Fukushima CAT3 08/12/2014 CENTER 08AZ000046 Seikagaku Corporation Takahagi Plant Takahagi Ibaraki CAT3 28/04/2011 08EZ280001 Thermo Fisher Diagnostics K.K. Logistics Center Ishioka Ibaraki CAT3 30/03/2015 08EZ280050 NISSUI PHARMACEUTICAL CO.,.LTD. YUKI PLANT Yuki Ibaraki CAT3 08/12/2014 08EZ280097 SEKISUI MEDICAL CO.,LTD. TSUKUBA PLANT Ryugasaki Ibaraki CAT 1, CAT 2, CAT3 08/12/2014 08EZ280110 KAINOS Laboratories, Inc. Kasama Factory Kasama Ibaraki CAT3 11/04/2019 09AZ200008 Chugai Pharma Manufacturing Co.,LTD. Utsunomiya Plant Utsunomiya Tochigi CAT 2 28/04/2011 09AZ200012 Maruha Nichiro Foods, Inc. Foods & Fine Chemicals Department Utsunomiya Tochigi CAT 2 28/04/2011 09EZ280056 INSTITUTE OF IMMUNOLOGY CO.,LTD. TOCHIGI PLANT Shimotsuke Tochigi CAT 2, CAT3 08/12/2014 09EZ286008 SB Bioscience Co., Ltd. Fujikura Kasei Co., Ltd. Sano Medical Sano Tochigi CAT 2, CAT3 08/12/2014 Satellite 1 / 4 Liste en vigueur Numéro Nom Ville Régions Activités Remarque Date de la demande d'agrément 11EZ200009 NICHIREI BIOSCIENCES INC. -
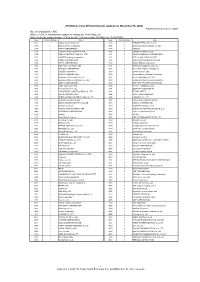
"JPX-Nikkei Index 400"
JPX-Nikkei Index 400 Constituents (applied on November 30, 2020) Published on November 9, 2020 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3086 1 J.FRONT RETAILING Co.,Ltd. 1333 1 Maruha Nichiro Corporation 3088 1 Matsumotokiyoshi Holdings Co.,Ltd. 1605 1 INPEX CORPORATION 3092 1 ZOZO,Inc. 1719 1 HAZAMA ANDO CORPORATION 3107 1 Daiwabo Holdings Co.,Ltd. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3116 1 TOYOTA BOSHOKU CORPORATION 1721 1 COMSYS Holdings Corporation 3141 1 WELCIA HOLDINGS CO.,LTD. 1766 1 TOKEN CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3167 1 TOKAI Holdings Corporation 1802 1 OBAYASHI CORPORATION 3197 1 SKYLARK HOLDINGS CO.,LTD. 1803 1 SHIMIZU CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1808 1 HASEKO Corporation 3288 1 Open House Co.,Ltd. 1812 1 KAJIMA CORPORATION 3289 1 Tokyu Fudosan Holdings Corporation 1820 1 Nishimatsu Construction Co.,Ltd. 3291 1 Iida Group Holdings Co.,Ltd. 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3349 1 COSMOS Pharmaceutical Corporation 1824 1 MAEDA CORPORATION 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1860 1 TODA CORPORATION 3382 1 Seven & I Holdings Co.,Ltd. 1861 1 Kumagai Gumi Co.,Ltd. 3391 1 TSURUHA HOLDINGS INC. 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3401 1 TEIJIN LIMITED 1881 1 NIPPO CORPORATION 3402 1 TORAY INDUSTRIES,INC. -

572KB/2Pages
Introduction/ Chapter I Chapter II Chapter III Chapter IV President’s Message Value Creation Story of MinebeaMitsumi Financial Strategy and Capital Policy Initiatives for Value Creation Initiatives to Support Value Creation List of Officers (As of August 2020) ■ Directors Attendance at the Board of Directors Meeting Attendance at the Board of Directors Meeting 100% (12/12) 100% (12/12) Representative Director, CEO & COO Representative Director, Vice Chairman Yoshihisa Kainuma Shigeru Moribe Apr. 1983 Member of Daini Tokyo Bar Association Mar. 1980 Joined MITSUMI ELECTRIC CO., LTD. Dec. 1988 Director, General Manager of Legal Department of the Company May 1990 General Manager of Development Headquarters, MITSUMI ELECTRIC CO., LTD. Sep. 1989 Member of New York State Bar Association Apr. 1991 Director, Head of Singapore branch, MITSUMI ELECTRIC CO., LTD. Dec. 1992 Managing Director and Deputy General Manager of Operations Headquarters Apr. 1994 Managing Director, MITSUMI ELECTRIC CO., LTD. Dec. 1994 Senior Managing Director, General Manager of European and American Regional Oct. 1999 Senior Managing Director, General Manager of Sales Headquarters, MITSUMI Sales Headquarters, Deputy General Manager of Operations Headquarters ELECTRIC CO., LTD. Jun. 2003 Director, Senior Managing Executive Officer Apr. 2002 Representative Director, President, MITSUMI ELECTRIC CO., LTD. Apr. 2009 Representative Director, President and Chief Executive Officer Jan. 2017 Adviser of the Company Jan. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. Apr. 2017 Director, Chairman of the Board of Directors, MITSUMI ELECTRIC CO., LTD. (Present) Jun. 2017 Representative Director, CEO & COO (Present) Jun. 2017 Representative Director, Vice Chairman (Present) Aug. 2019 Representative Director, Chairman of the Board of Directors, U-Shin Ltd.