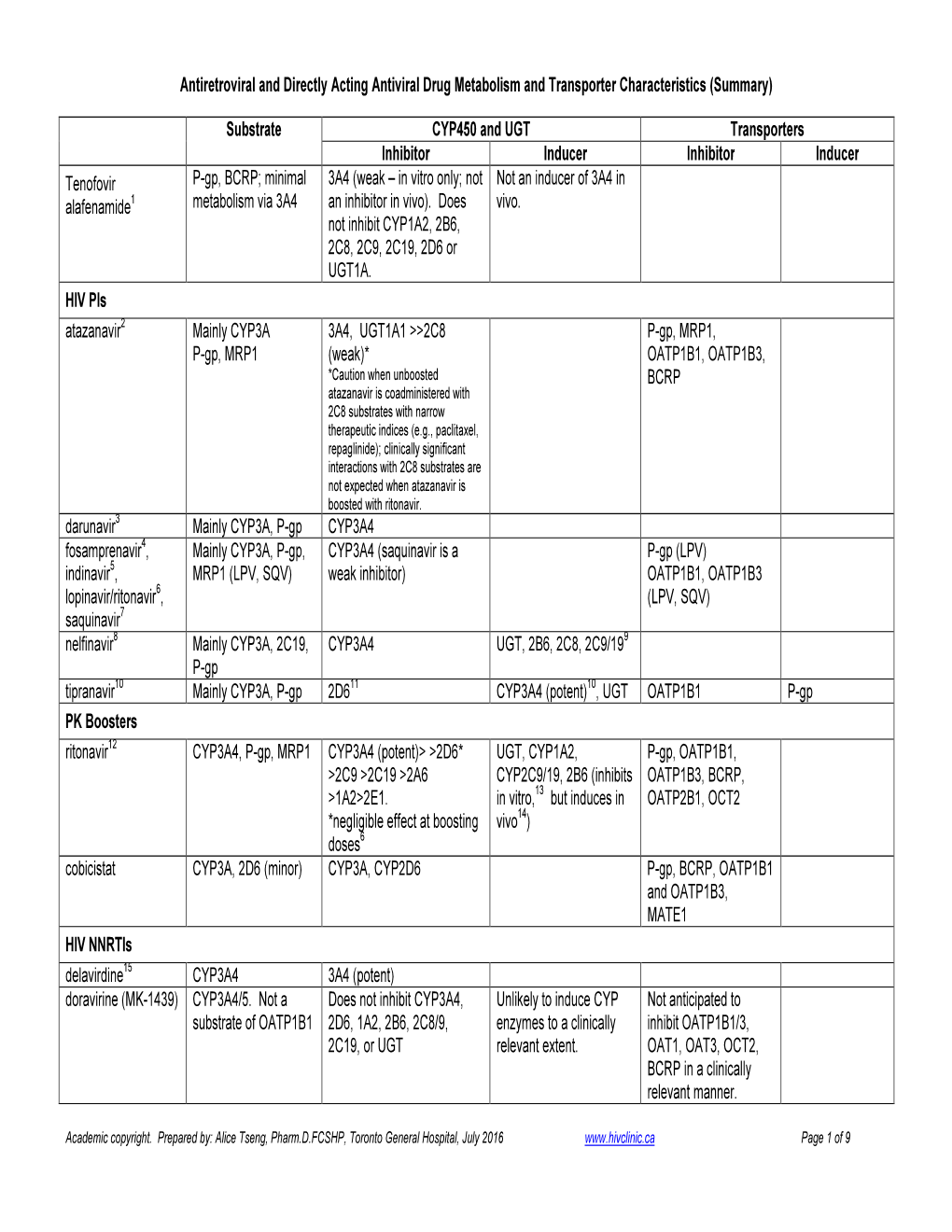
(Summary) Substrate CYP450 And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hepatitis-C-Virus-Induced Micrornas Dampen Interferon-Mediated
LETTERS Hepatitis-C-virus-induced microRNAs dampen interferon-mediated antiviral signaling Abigail Jarret1, Adelle P McFarland1,6,7, Stacy M Horner1,6,7, Alison Kell1, Johannes Schwerk1, MeeAe Hong1, Samantha Badil1, Rochelle C Joslyn1, Darren P Baker2,6, Mary Carrington3,4, Curt H Hagedorn5, Michael Gale Jr1 & Ram Savan1 Hepatitis C virus (HCV) infects 200 million people globally, currently in clinical trials6,10. More recently, interferon-free com- and 60–80% of cases persist as a chronic infection that will binations of HCV protease and RNA polymerase inhibitors, some- progress to cirrhosis and liver cancer in 2–10% of patients1–3. times with ribavirin, have produced dramatic results in a subset of We recently demonstrated that HCV induces aberrant expression patients10. However, the emergence of resistant HCV variants and of two host microRNAs (miRNAs), miR-208b and miR-499a-5p, the high cost of DAA treatments might limit the availability of these encoded by myosin genes in infected hepatocytes4. These therapies worldwide11–13. The usefulness of DAA-based therapies for miRNAs, along with AU-rich-element-mediated decay, suppress some patients—such as those with advanced infection, who are at IFNL2 and IFNL3, members of the type III interferon (IFN) the highest risk for hepatic decompensation, liver failure, hepato- gene family, to support viral persistence. In this study, we cellular carcinoma and/or death—also remains to be fully proven13. show that miR-208b and miR-499a-5p also dampen type I Hence, it is critical to identify the mechanisms that HCV employs IFN signaling in HCV-infected hepatocytes by directly which lead to the failure of type I IFN therapy. -

Hepatitis C Agents Therapeutic Class Review
Hepatitis C Agents Therapeutic Class Review (TCR) November 2, 2018 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004–2018 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Mfr FDA-Approved Indications Interferons peginterferon alfa-2a Genentech Chronic hepatitis C (CHC) 1 (Pegasys®) . -

Sofosbuvir-Velpatasvir (Epclusa)
© Hepatitis C Online PDF created September 29, 2021, 4:07 am Sofosbuvir-Velpatasvir (Epclusa) Table of Contents Sofosbuvir-Velpatasvir Epclusa Summary Drug Summary Adverse Effects Class and Mechanism Manufacturer for United States Indications Contraindications Dosing Clinical Use Cost and Medication Access Resistance Key Drug Interactions Full Prescribing Information Figures Drug Summary Sofosbuvir-velpatasvir is a pangenotypic NS5A-NS5B inhibitor single-pill combination regimen that has potent activity against hepatitis C virus (HCV) genotypes 1, 2, 3, 4, 5, and 6. It provides a much-needed option for patients with HCV genotype 3 infection, including those with compensated cirrhosis. Notably, an abbreviated duration of 8 weeks has not been studied with sofosbuvir-velpatasvir for any of the genotypes, except in conjunction with a third agent (voxilaprevir). Sofosbuvir-velpatasvir, like ledipasvir-sofosbuvir, may have have levels significantly reduced with acid-reducing agents, particularly proton-pump inhibitors. Adverse Effects The most common adverse effects, observed in at least 10% of phase 3 trial participants, were headache and fatigue. Class and Mechanism Sofosbuvir-Velpatasvir is an oral fixed-dose combination of sofosbuvir, a nucleotide analog NS5B polymerase inhibitor and velapatasvir, an NS5A replication complex inhibitor. Sofosbuvir is currently approved in the Page 1/4 United States for the treatment of genotype 1, 2, 3 and 4 HCV infection with different regimens and durations dependent on the HCV genotype. Velpatasvir (formerly GS-5816) is a novel NS5A inhibitor that has potent in vitro anti-HCV activity across all genotypes at the picomolar level. The combination of sofosbuvir-velpatasvir is the first once-daily single-tablet regimen with pangenotypic activity. -

Hepatitis C Viral RNA Genotype 1 NS5B Drug Resistance
Test Summary TM Hepatitis C Viral RNA Genotype 1 NS5B Drug Resistance The Hepatitis C Viral RNA Genotype 1 NS5B Drug Resistance Test Code: 906679 assay determines the HCV genotype (1a, 1b, or 1) and detects mutations associated with resistance to sofosbuvir. The Specimen Requirements: 2 mL frozen plasma from an identication of specic mutations or polymorphisms may be EDTA lavender-top tube (0.6 mL minimum). useful to optimize treatment selection. INDIVIDUALS SUITABLE FOR TESTING CPT Code*: 87902 • Individuals with genotype 1 HCV infection who experience treatment failure with sofosbuvir CLINICAL USE METHOD • Identify resistance-associated mutation as potential • Reverse transcription polymerase chain reaction (PCR) cause of NS5B inhibitor (sofosbuvir) failure and DNA sequencing of NS5B codons 270 to 530 • Analytical sensitivity: >95% for viral loads ≥700 IU/mL CLINICAL BACKGROUND Results reported: HCV infection aects more than 3.5 million individuals in the • United States.1 Left untreated, it can lead to progressive liver – HCV genotype: 1a, 1b, 1, or not detected (genotypes injury, cirrhosis, hepatocellular carcinoma, and the need for other than 1 may not be detected) liver transplantation. Of the 6 major HCV genotypes, genotype – Sofosbuvir resistance: predicted or not predicted 1 (including subtypes 1a and 1b) is the most prevalent by far in the United States.2 Combination therapy with pegylated INTERPRETIVE INFORMATION interferon (PEG) plus ribavirin was the mainstay of treatment An interpretation of “resistance predicted” suggests that for all genotypes, but resulted in low sustained virologic treatment failure with sofosbuvir may be due to the presence response rates of approximately 40% to 50% in HCV genotype of an NS5B mutation (S282T). -

Hepatitis C Virus RNA-Dependent RNA-Polymerases from Genotypes 1 to 5
De Novo Polymerase Activity and Oligomerization of Hepatitis C Virus RNA-Dependent RNA-Polymerases from Genotypes 1 to 5 Pilar Clemente-Casares1., Alberto J. Lo´ pez-Jime´nez1,2., Itxaso Bello´ n-Echeverrı´a1, Jose´ Antonio Encinar4, Elisa Martı´nez-Alfaro2, Ricardo Pe´rez-Flores3, Antonio Mas1* 1 Centro Regional de Investigaciones Biome´dicas (CRIB), Universidad de Castilla La Mancha, Albacete, Spain, 2 Infectious Disease Unit, Complejo Hospitalario Universitario de Albacete, Albacete, Spain, 3 Digestive Department, Complejo Hospitalario Universitario de Albacete, Albacete, Spain, 4 Instituto de Biologı´a Molecular y Celular, Universidad Miguel Herna´ndez, Elche, Spain Abstract Hepatitis C virus (HCV) shows a great geographical diversity reflected in the high number of circulating genotypes and subtypes. The response to HCV treatment is genotype specific, with the predominant genotype 1 showing the lowest rate of sustained virological response. Virally encoded enzymes are candidate targets for intervention. In particular, promising antiviral molecules are being developed to target the viral NS3/4A protease and NS5B polymerase. Most of the studies with the NS5B polymerase have been done with genotypes 1b and 2a, whilst information about other genotypes is scarce. Here, we have characterized the de novo activity of NS5B from genotypes 1 to 5, with emphasis on conditions for optimum activity and kinetic constants. Polymerase cooperativity was determined by calculating the Hill coefficient and oligomerization through a new FRET-based method. The Vmax/Km ratios were statistically different between genotype 1 and the other genotypes (p,0.001), mainly due to differences in Vmax values, but differences in the Hill coefficient and NS5B oligomerization were noted. -

Reviews in Basic and Clinical Gastroenterology
GASTROENTEROLOGY 2010;138:447–462 REVIEWS IN BASIC AND CLINICAL GASTROENTEROLOGY REVIEWS IN BASIC AND CLINICAL John P. Lynch and David C. Metz, Section Editors GASTROENTEROLOGY Resistance to Direct Antiviral Agents in Patients With Hepatitis C Virus Infection CHRISTOPH SARRAZIN and STEFAN ZEUZEM J. W. Goethe-University Hospital, Medizinische Klinik 1, Frankfurt am Main, Germany Chronic hepatitis C virus (HCV) infection is one of agents that are also named specific, targeted antiviral the major causes of cirrhosis, hepatocellular carci- therapies (STAT-C) for HCV infection are in phase 1–3 noma, and liver failure that leads to transplantation. trials. We review resistance to DAA agents, focusing on The current standard treatment, a combination of compounds in development such as reagents that target pegylated interferon alfa and ribavirin, eradicates the the HCV nonstructural (NS)3 protease, the NS5A pro- virus in only about 50% of patients. Directly acting tein, and the RNA-dependent RNA polymerase NS5B. We antiviral (DAA) agents, which inhibit HCV replica- also discuss indirect inhibitors or compounds that in- tion, are in phase 1, 2, and 3 trials; these include hibit HCV replication by not yet completely resolved reagents that target the nonstructural (NS)3 protease, mechanisms, such as cyclophilin inhibitors, nitazox- the NS5A protein, the RNA-dependent RNA-polymer- anide, and silibinin (Table 1, Figure 1). ase NS5B, as well as compounds that directly inhibit HCV replication through interaction with host cell Parameters That Affect Resistance proteins. Because of the high genetic heterogeneity of Heterogeneity of HCV HCV and its rapid replication, monotherapy with HCV has a high rate of turnover; its half-life was DAA agents poses a high risk for selection of resistant estimated to be only 2–5 hours, with the production and variants. -

A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity
Journal of Clinical Medicine Article A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity Jung Sun Min 1,2, Geon-Woo Kim 1,2, Sunoh Kwon 1,2,* and Young-Hee Jin 2,3,* 1 Herbal Medicine Research Division, Korea Institute of Oriental Medicine, Daejeon 34054, Korea; [email protected] (J.S.M.); [email protected] (G.-W.K.) 2 Center for Convergent Research of Emerging Virus Infection, Korea Research Institute of Chemical Technology, Daejeon 34114, Korea 3 KM Application Center, Korea Institute of Oriental Medicine, Daegu 41062, Korea * Correspondence: [email protected] (S.K.); [email protected] (Y.-H.J.); Tel.: +82-42-868-9675 (S.K.); +82-42-610-8850 (Y.-H.J.) Received: 25 June 2020; Accepted: 20 July 2020; Published: 27 July 2020 Abstract: Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19) are emerging zoonotic diseases caused by coronavirus (CoV) infections. The viral RNA-dependent RNA polymerase (RdRp) has been suggested as a valuable target for antiviral therapeutics because the sequence homology of CoV RdRp is highly conserved. We established a cell-based reporter assay for MERS-CoV RdRp activity to test viral polymerase inhibitors. The cell-based reporter system was composed of the bicistronic reporter construct and the MERS-CoV nsp12 plasmid construct. Among the tested nine viral polymerase inhibitors, ribavirin, sofosbuvir, favipiravir, lamivudine, zidovudine, valacyclovir, vidarabine, dasabuvir, and remdesivir, only remdesivir exhibited a dose-dependent inhibition. Meanwhile, the Z-factor and Z0-factor of this assay for screening inhibitors of MERS-CoV RdRp activity were 0.778 and 0.782, respectively. -

Follow-Up Results of HCV GT2 Patients
ANTICANCER RESEARCH 39 : 3855-3862 (2019) doi:10.21873/anticanres.13535 Follow-up Results of HCV GT2 Patients After Sofosbuvir/Ribavirin Therapy: Careful Attention to Occurrence of HCC TOMOHIRO KANEKO 1, TATSUO KANDA 1, KAZUSHIGE NIREI 1, NAOKI MATSUMOTO 1, MOTOMI YAMAZAKI 1, TOSHIKATSU SHIBATA 1, AKINORI TAMURA 1, MASAHIRO OGAWA 1, NORIKO NAKAJIMA 1, SHUNICHI MATSUOKA 1, KAZUMICHI KURODA 1, TOMOE KOMORIYA 2, TATSUO YAMAMOTO 3, TADATOSHI TAKAYAMA 4 and MITSUHIKO MORIYAMA 1 1Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan; 2Department of Sustainable Engineering, College of Industrial Technology, Nihon University, Chiba, Japan; 3Department of Obstetrics and Gynecology, Nihon University School of Medicine, Tokyo, Japan; 4Department of Digestive Surgery, Nihon University School of Medicine, Tokyo, Japan Abstract. Background: We examined treatment the efficacy Chronic hepatitis C virus (HCV) infection is a major cause of and data on long-term outcomes in real-world Japanese hepatocellular carcinoma (HCC) and end-stage liver disease patients infected with hepatitis C virus (HCV) genotype 2 in Japan and Southern Europe (1, 2). Recent interferon-free treated with 12-week sofosbuvir/ribavirin combination therapy. therapy with direct-acting antivirals (DAAs) against HCV Patients and Methods: In a total of 86 patients who were treated resulted in higher sustained virological response (SVR) rates with sofosbuvir/ribavirin, sustained virological response (SVR) with shorter treatment durations and few adverse events (3). rates and long-term-outcomes were retrospectively analyzed. In Japan, the estimated proportion of the general Results: The adherence to this combination therapy was 98.8%. population with HCV infection is 1.0-2.0%, and HCV The rates of SVR at week 24 (SVR24) achieved with this genotype 2 (GT2) accounts for 30% of chronic HCV treatment according to the ‘intention-to-treat’ and ‘per-protocol’ infections (4). -

IFNL4-ΔG Genotype Is Associated with Slower Viral Clearance In
BRIEF REPORT IFNL4-ΔG Genotype Is Associated In treatment-naive hepatitis C virus (HCV) genotype-1 pa- tients, the current standard-of-care treatment, an NS3 prote- With Slower Viral Clearance in ase inhibitor with IFN-α/RBV, results in sustained virological Hepatitis C, Genotype-1 Patients response (SVR) rates of 63%–75% [1]. However, many pa- tients remain untreated due to relative or absolute contraindi- Treated With Sofosbuvir and cations, fear of adverse events, or pill burden. Treatment of Ribavirin chronic HCV infection is evolving towards IFN-α–free, direct-acting antiviral (DAA) regimens that specifically target Eric G. Meissner,1,a Dimitra Bon,2,a Ludmila Prokunina-Olsson,3 the HCV protease (NS3/4A), RNA polymerase (NS5B), or 3 4 ’ 5 2 Wei Tang, Henry Masur, Thomas R. O Brien, Eva Herrmann, nonstructural protein NS5A. While phase 2 and 3 studies of Shyamasundaran Kottilil,1 and Anuoluwapo Osinusi1,6,7 DAA therapies demonstrate promising efficacy and tolerabili- 1Laboratory of Immunoregulation/National Institute of Allergy and Infectious Diseases/National Institutes of Health, Bethesda, Maryland; 2Institute of ty [2], issues of cost and access to treatment will remain rele- Biostatistics and Mathematical Modeling, Johann Wolfgang Goethe University, vant. Identification of host factors that affect differential 3 Frankfurt, Germany; Laboratory of Translational Genomics, Division of Cancer response to DAA therapies could permit personalization of Epidemiology and Genetics/National Cancer Institute/National Institutes of Health, HCV -

Development of a Multiplex Phenotypic Cell-Based High Throughput Screening Assay to Identify Novel Hepatitis C Virus Antiviralsq
Antiviral Research 99 (2013) 6–11 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Short Communication Development of a multiplex phenotypic cell-based high throughput screening assay to identify novel hepatitis C virus antivirals q Hee-Young Kim a, Xiaolan Li b, Christopher T. Jones c, Charles M. Rice c, Jean-Michel Garcia a, ⇑ Auguste Genovesio b, Michael A.E. Hansen b, Marc P. Windisch a, a Applied Molecular Virology, Institut Pasteur Korea, 696 Sampyeong-dong, Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea b Image Mining, Institut Pasteur Korea, 696 Sampyeong-dong, Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea c Center for the Study of Hepatitis C, Laboratory of Virology and Infectious Disease, Rockefeller University, NY, USA article info abstract Article history: Hepatitis C virus (HCV) infection is a global health concern with chronic liver damage threatening 3% of Received 5 February 2013 the world’s population. To date, the standard of care is a combination of pegylated interferon-alpha with Revised 24 April 2013 ribavirin, and recently two direct acting antivirals have entered the clinics. However, because of side Accepted 27 April 2013 effects, drug resistance and viral genotype-specific differences in efficacy current and potentially also Available online 6 May 2013 future therapies have their limitations. Here, we describe the development of a phenotypic high-through- put assay to identify new cross-genotype inhibitors with novel mechanism of action, by combining a Keywords: genotype (gt) 1 replicon with the infectious HCV gt2 cell culture system. To develop this phenotypic HCV multiplex assay, HCV reporter cells expressing RFP-NLS-IPS and gt1b replicon cells expressing multiplex phenotypic NS5A-GFP were co-plated and treated with compounds followed by inoculation with gt2a HCV. -

02-10-7769 KBH NKF HCV Clin Bulletin V2.Indd
Managing Hepatitis C and CKD: A Clinical Update > Introduction > Associations Between HCV and CKD > Overview of HCV Evaluation > New Options in HCV Management > Summary Introduction Overview of HCV Evaluation Hepatitis C virus (HCV) is the most common Diagnostic tests consist of two broad categories: chronic blood-borne infection in the United States (1) serologic assays that detect antibodies to HCV, (U.S.) and is more prevalent among patients with and (2) molecular assays that detect or quantify chronic kidney disease (CKD) than in the general HCV RNA.12 The Centers for Disease Control and population.1,2,3 HCV is usually asymptomatic, and Prevention (CDC) recommends a testing sequence detection requires screening patients with risk for identifying current HCV infection, consisting factors and testing for serum HCV antibodies or HCV of initial HCV antibody testing (either rapid or RNA (viral load). The field of HCV has dramatically laboratory-conducted assay) followed by an HCV changed in recent years with the advent of direct- RNA assay for all positive antibody tests.4,13 acting antivirals (DAAs) and multi-class drug Several organizations have provided guidelines for combinations. These newer therapies have been which individuals should be tested. All guidelines the focus of clinical research in recent years for their generally recommend screening patients at improved efficacy (90-100% sustained viral response increased risk for HCV (Table 1).4,13,14 Testing of (SVR) in most cases), with improved side effect patients with CKD should be considered in all profiles and better tolerability. Recently approved patients on dialysis and in CKD patients with DAA regimens can safely and effectively treat unexplained proteinuria, microscopic hematuria, patients with CKD and patients on dialysis. -

Interferon-Combination Strategies for the Treatment of Chronic Hepatitis C
30 Interferon-Combination Strategies for the Treatment of Chronic Hepatitis C Andrew Aronsohn, MD1 Donald Jensen, MD1 1 Center for Liver Disease, Section of Gastroenterology, Hepatology Address for correspondence Andrew Aronsohn, MD, Center for Liver and Nutrition, University of Chicago Medical Center, Chicago, Illinois Disease, Section of Gastroenterology, Hepatology and Nutrition, 5841 South Maryland Avenue, MC 7120, Chicago, IL 60637 Semin Liver Dis 2014;34:30–36. (e-mail: [email protected]). Abstract Direct acting antiviral agents have revolutionized hepatitis C (HCV) therapy. Many agents that are either currently available or undergoing investigation offer higher rates of sustained virologic response, reduced toxicity and shorter duration of therapy when compared to traditional treatment consisting of pegylated interferon and ribavirin. Keywords Although interferon free therapy may be a preferred option, some patients may still ► hepatitis C require an interferon based regimen to ensure efficacy. In this review, we discuss ► hepatitis C therapy therapeutic strategies which utilize various combinations of protease inhibitors, NS5A ► direct-acting antiviral inhibitors, nucleotide polymerase inhibitors and non-nucleoside polymerase inhibitors agents along with pegylated interferon in the treatment of chronic HCV. In 2011, the first direct-acting antiviral agents (DAAs) became complex is involved in decreasing activity of type 1 IFN available for use in the treatment of chronic hepatitis C. In though cleavage of IFN-B promoter