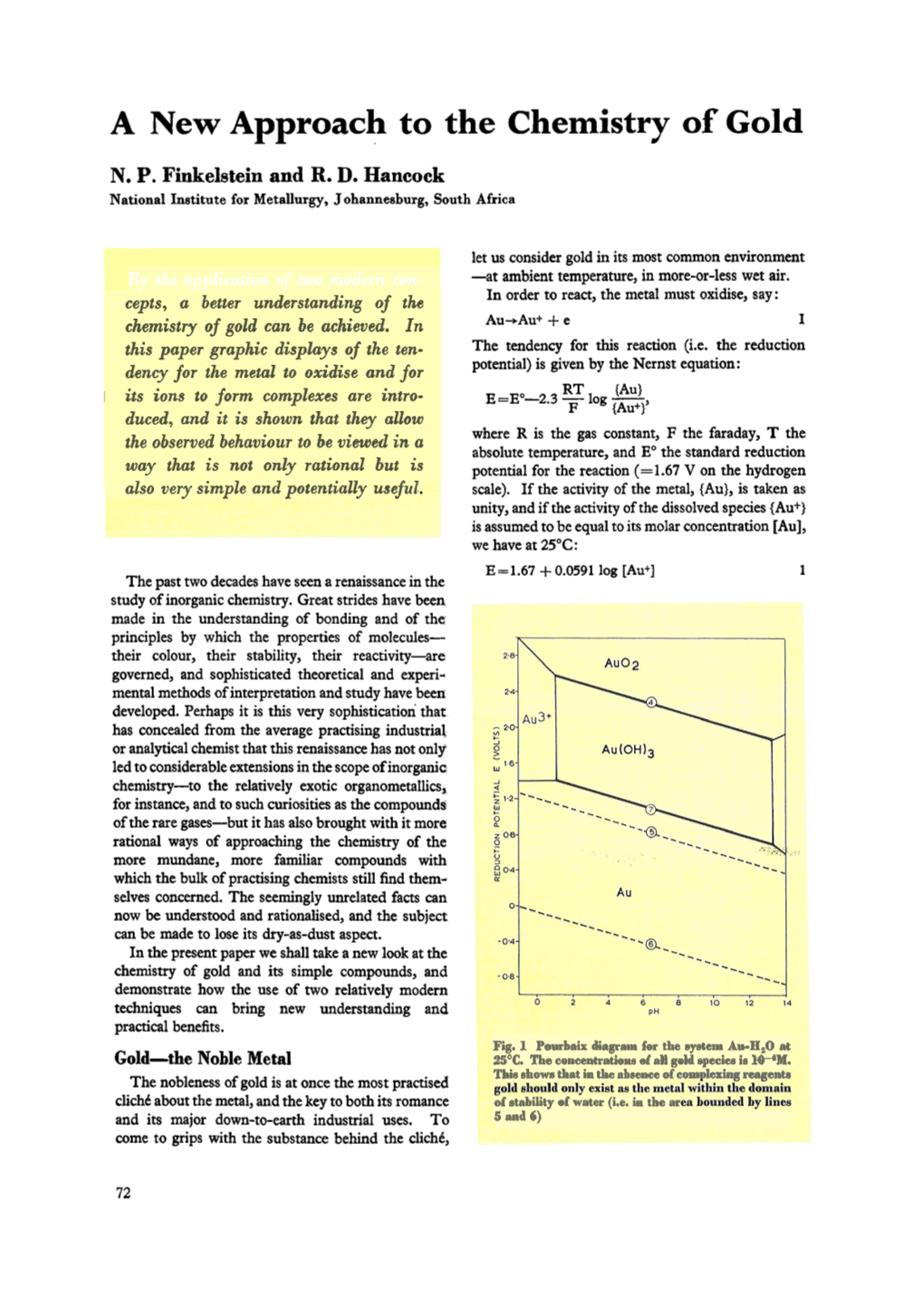
A New Approach to the Chemistry of Gold
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers
energies Review Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers Ana L. Santos 1,2, Maria-João Cebola 3,4,5 and Diogo M. F. Santos 2,* 1 TecnoVeritas—Serviços de Engenharia e Sistemas Tecnológicos, Lda, 2640-486 Mafra, Portugal; [email protected] 2 Center of Physics and Engineering of Advanced Materials (CeFEMA), Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisbon, Portugal 3 CBIOS—Center for Research in Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisbon, Portugal; [email protected] 4 CERENA—Centre for Natural Resources and the Environment, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon, Portugal 5 Escola Superior Náutica Infante D. Henrique, 2770-058 Paço de Arcos, Portugal * Correspondence: [email protected] Abstract: Environmental issues make the quest for better and cleaner energy sources a priority. Worldwide, researchers and companies are continuously working on this matter, taking one of two approaches: either finding new energy sources or improving the efficiency of existing ones. Hydrogen is a well-known energy carrier due to its high energy content, but a somewhat elusive one for being a gas with low molecular weight. This review examines the current electrolysis processes for obtaining hydrogen, with an emphasis on alkaline water electrolysis. This process is far from being new, but research shows that there is still plenty of room for improvement. The efficiency of an electrolyzer mainly relates to the overpotential and resistances in the cell. This work shows that the path to better Citation: Santos, A.L.; Cebola, M.-J.; electrolyzer efficiency is through the optimization of the cell components and operating conditions. -

Pourbaix Diagrams Pe = E/0.0592 E Is the Redox Potential Vs
Oxidation-Reduction Reactions Oxidation Reduction Oxidation-Reduction Reactions Oxidation Reduction Half Cell Potentials ECe EFe Half Cell Potentials are always written as reductions: Since we always use half cell differences, we can add an arbitrary constant to all half cells. By convention, we assume that the Eo for the normal hydrogen electrode (NHE) is equal to zero: Half Cell Reaction for Hydrogen: Half Cell Potentials ECe EFe Half Cell Potentials are always written as reductions: E°(Fe) = +0.77 V E°(Ce) = +1.61 V E°(Cell) = E°(Fe) - E°(Ce) = - 0.91 V Spontaneous? Half Cell Potentials ECe EFe Half Cell Potentials are always written as reductions: E°(Fe) = +0.77 V E°(Ce) = +1.61 V E°(Cell) = E°(Fe) - E°(Ce) = - 0.91 V Not Spontaneous - Electrolytic Cell! The reverse reaction is spontaneous - Galvanic Cell. Redox Titrations Ce4+ Fe2+ Ce4+ + Fe2+ = Ce3+ + Fe3+ This reaction is spontaneous! UC Davis chemwiki. Electrochemical Alpha Fractions EFe is called the Redox Potential Electrolysis of Water At the positively charged anode, an oxidation reaction occurs, generating oxygen gas (these equations are for acidic pH): + Oxidation at anode: 2 H2O(l) → O2(g) + 4 H (aq) + 4e At the negatively charged cathode, a reduction reaction occurs, generating hydrogen gas: + − Reduction at cathode: 2 H (aq) + 2e → H2(g) Half Cell Potentials: + − O2(g) + 4 H (aq) + 4e → 2 H2O(l) E° = +1.229 V + − 2 H (aq) + 2e → H2(g) E° = -0.8277 V E°(cell) = E°(red) - E°(ox) = -0.8277 – 1.229 = -2.056 V Spontaneous? Reduction is on the left in this picture (sorry) Electrolysis -

Water Electrolysis at the Thermodynamic Limit Matthew D
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2007 Water Electrolysis at the Thermodynamic Limit Matthew D. Merrill Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES WATER ELECTROLYSIS AT THE THERMODYNAMIC LIMIT By MATTHEW D. MERRILL A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctorate of Philosophy Degree Awarded: Fall Semester, 2007 The members of the Committee approve the Dissertation of Matthew D. Merrill defended on December 12, 2006. Robert C. Dougherty Professor Directing Dissertation Anjaneyulu Krothapalli Outside Committee Member Timothy M. Logan Committee Member Nancy L. Greenbaum Committee Member Kenneth A. Goldsby Committee Member Approved: Joseph B. Schlenoff, Chair Department of Chemistry and Biochemistry Joseph Travis, Dean College of Arts and Sciences The Office of Graduate Studies has verified and approved the above name committee members. ii This work is dedicated to my Father, who asked me to knock and then opened the door. To anyone and everyone to whom this work will be of use. ii ACKNOWLEDGEMENTS I would first like to acknowledge my gratitude to my parents and brother. The older I get, the more I understand and value the blessings you have given me. To all my family, you have provided such strong and nurturing roots. Dani, my beautiful wife, your love and support is amazing. And to my friends, thank you too for helping to get me where I am today and preparing me for tomorrow. -

Assessment of Nickel-Based Alloy Passive Film Stability Through Thermodynamic Modeling
'WRC Form 390A U.S. NUCLEAR REGULATORY COMMISSION ( 12-2003) hsIRCMD 3.9 RELEASE TO PUBLISH UNCLASSIFIED NRC CONTRACTOR SPEECHES, PAPERS, AND JOURNAL ARTICLES (Please type or prfnt) 1 TITLE (State in full as it appears on the speech, paper, or journal article) Assessment of Nickel-Based Alloy Passive Film Stability T9rough Thermodynamic Modeling 2. AUTHOR(S) P. Shukla, R. Pabalan, and L. Yang 3. NAME OF CONFERENCE, LOCATION, AND DATE(s) NACE 2009 Annual Conference & Exposition, Atlanta, Georgia, March 22-26, 2009 4. NAME OF PUBLICATION NACE Conference Proceedings 5. NAME AND ADDRESS OF THE PUBLISHER TELEPHONE NUMBER OF THE PUBLISHER NICE International (281) 228-6200 1440 South Creek Drive Houston, TX 77084-4906 6. CONTRACTOR NAME AND COMPLETE MAILING ADDRESS TELEPHONE NUMBER OF THE CONTRACTOR (Include ZIP code) Dr Pavan Shukla (210) 522-6534 Center for Nuclear Waste Regulatory Analyses Southwest Research Institute 6220 Culebra Road Sail Antonio, TX 78238 7. CERTIFICATION YfiS NO (ANSWER ALL QUESTIONS) X A. COPYRIGHTED MATERIAL - Does this speech, paper, or journal article contain copyrighted material: If yes, attach a letter of release from the source that holds the copyright. X 6. PATENT CLEARANCE - Does this speech, paper, or journal article require patent clearance? If yes, the NRC Patent Counsel must signify clearance by signing below. NRC PATENT COUNSEL SIGNATURE (Type or Print Name) C. REFERENCE AVAILABILITY - Is all material referenced in this speech, paper, or journal article available to the public either through a public library, the Government Printing Office, the followed by the English units in brackets, pursuant to the NRC Policy Statement implementing the Omnibus Trade and Competitiveness Act of 1988. -

Pourbaix Diagram Notes by MIT Student (And MZB)
Pourbaix Diagram Notes by MIT Student (and MZB) 1. Battery equilibrium electrochemistry Suppose an electrochemical reaction happening on the electrode/electrolyte interface: − R O + ne (1) At equilibrium, the electrode potential is related to activities of the reactants by the Nernst equa tion: kT a an 0 O e E = E + ln (2) ne aR 0 where E is the electrical potential of the electrode measured against a standard hydrogen cell electrode under standard conditions. aO is the chemical activity of the oxidized state, aR the n aO ae chemical activity of the reduced state. At equilibrium, the reaction constant KO = is thus aR determined by the electrode potential: ne 0 KO = exp (E − E ) kT Given the dependence of chemical activities on other thermodynamic parameters, the equilibrium electrode potential will separate the oxidized/reduced phases on a phase diagram. If the electrode potential is raised above the equilibrium potential, oxidization is preferred. If below the equilibrium potential, reduction is preferred. 2. Pourbaix diagram One certain class of phase diagrams is displayed by the equilibrium electrical potential plotted against the pH, when some of the reactants have chemical activities that vary with pH. For instance + if H is part of the electrode reaction, then under dilute approximation, aH+ ∼ cH+ . The pH of + the electrolyte is defined as the negative of the natural log of the H ion concentration in the electrolyte: pH = −log10cH+ (3) Under room temperature, water as the most common solvant by self-ionization will give pH = 7 which is the neutral pH. For an acidic solution pH < 7, for basic pH > 7. -

Iron Sulfide Formation on Iron Substrates by Electrochemical
Article Cite This: Cryst. Growth Des. XXXX, XXX, XXX-XXX pubs.acs.org/crystal Iron Sulfide Formation on Iron Substrates by Electrochemical Reaction in Anoxic Conditions † ‡ # ‡ † ‡ Xiaofei Guan, , , Brandon C. Enalls, David R. Clarke,*, and Peter Girguis*, † ‡ John A. Paulson School of Engineering and Applied Sciences and Department of Organismic & Evolutionary Biology, Harvard University, Cambridge, Massachusetts 02138, United States *S Supporting Information ABSTRACT: Electrodeposition has been widely used for the low-cost formation of large-area thin films of various crystalline materials. In this work, we use thermodynamic calculations to guide electrochemical experiments, in which we conduct a systematic investigation of crystalline iron sulfides formation on electrically poised iron substrates in H2S aqueous solutions. Whereas thermodynamic calculations predict the formation of fi iron pyrite (FeS2) lms at the conditions tested, the principal iron−sulfur mineral phases observed were mixtures of fi mackinawite (Fe1+xS) and cubic iron sul de (phase identi- fication and characterization included X-ray diffraction, Raman spectroscopy, scanning electron microscopy, and transmission electron microscopy). These data suggest that the phases formed are determined kinetically, not thermodynamically, across all tested electrodeposition conditions. The results are compared with the formation of iron sulfides produced by the “sour corrosion” of iron and steels, and in addition the effects of the experimental variables on the formation and morphologies of crystalline iron sulfides are discussed. 1. INTRODUCTION In contrast to the biogenic formation of iron pyrite, its Iron and sulfur are among the most abundant elements in formation during metal corrosion is much better understood, including the conditions under which nonstoichiometric Earth’s crust. -

Pourbaix Diagrams at Elevated Temperatures ~A Study of Zn and Sn~
Pourbaix Diagrams at Elevated Temperatures ~A Study of Zn and Sn~ by Olga Palazhchenko A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Materials Science in The Faculty of Energy Systems and Nuclear Science Materials Science University of Ontario Institute of Technology August 2012 © Olga Palazhchenko, 2012 Abstract Metals in industrial settings such as power plants are often subjected to high temperature and pressure aqueous environments, where failure to control corrosion compromises worker and environment safety. For instance, zircaloy (1.2-1.7 wt.% Sn) fuel rods are exposed to aqueous 250-310 oC coolant in CANDU reactors. The Pourbaix (EH-pH) diagram is a plot of electrochemical potential versus pH, which shows the domains of various metal species and by inference, corrosion susceptibility. Elevated temperature data for tin +II and tin +IV species were obtained using solid-aqueous phase equilibria with the respective oxides, in a batch vessel with in-situ pH measurement. Solubilities, determined via spectroscopic techniques, were used to calculate equilibrium constants and the Gibbs energies of Sn complexes for E-pH diagram construction. The 3+ SnOH and SnOH species were incorporated, for the first time, into the 298.15 K and 358.15 K diagrams, with novel G values determined at 358.15 K. Key words: Pourbaix diagrams, EH-pH, elevated temperatures, solubility, equilibrium, metal oxides, hydrolysis, redox potential, pH, thermochemical data, tin, zinc, zircaloy, corrosion, passivity. iii Acknowledgements To start, thank you to my thesis supervisor, Dr. Matthew Kaye, for the tremendous support over the past two years. Thank you for always making me feel that I could indeed accomplish this seemingly insurmountable task. -

An Electrochemical Characterization of a Vanadium-Based Deoxydehydration Catalyst Joel Eric Baker University of Arkansas, Fayetteville
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2017 An Electrochemical Characterization of a Vanadium-based Deoxydehydration Catalyst Joel Eric Baker University of Arkansas, Fayetteville Follow this and additional works at: https://scholarworks.uark.edu/etd Part of the Analytical Chemistry Commons, and the Inorganic Chemistry Commons Recommended Citation Baker, Joel Eric, "An Electrochemical Characterization of a Vanadium-based Deoxydehydration Catalyst" (2017). Theses and Dissertations. 1922. https://scholarworks.uark.edu/etd/1922 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. An Electrochemical Characterization of a Vanadium-based Deoxydehydration Catalyst A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemistry by Joel E. Baker Missouri Southern State University Bachelor of Science in Chemistry, 2014 May 2017 University of Arkansas This thesis is approved for recommendation to the Graduate Council. __________________________________ Dr. Stefan Kilyanek Thesis Director __________________________________ Dr. David Paul Committee Member __________________________________ __________________________________ Dr. Ryan Tian Dr. Bill Durham Committee Member Committee Membe Abstract Alternative methods for the conversion of polyols into olefins, be it for carbon storage or hydrocarbon fuel production, have become prevalent in today’s chemical industry. One process in particular, deoxydehydration (DODH) has been proven effective in taking sustainable biomass derivatives and converting them through the utilization of various homogenous metal catalysts. While this process may show productive yields and material conversion, it is hindered by the need of a sacrificial reductant. -
Predicting Aqueous Stability of Solid with Computed Pourbaix Diagram Using SCAN Functional ✉ Zhenbin Wang 1, Xingyu Guo2, Joseph Montoya3 and Jens K
www.nature.com/npjcompumats ARTICLE OPEN Predicting aqueous stability of solid with computed Pourbaix diagram using SCAN functional ✉ Zhenbin Wang 1, Xingyu Guo2, Joseph Montoya3 and Jens K. Nørskov 1 In this work, using the SCAN functional, we develop a simple method on top of the Materials Project (MP) Pourbaix diagram framework to accurately predict the aqueous stability of solids. We extensively evaluate the SCAN functional’s performance in computed formation enthalpies for a broad range of oxides and develop Hubbard U corrections for transition-metal oxides where the standard SCAN functional exhibits large deviations. The performance of the calculated Pourbaix diagram using the SCAN functional is validated with comparison to the experimental and the MP PBE Pourbaix diagrams for representative examples. Benchmarks indicate the SCAN Pourbaix diagram systematically outperforms the MP PBE in aqueous stability prediction. We further show applications of this method in accurately predicting the dissolution potentials of the state-of-the-art catalysts for oxygen evolution reaction in acidic media. npj Computational Materials (2020) 6:160 ; https://doi.org/10.1038/s41524-020-00430-3 INTRODUCTION improvement accuracy in both energetics and geometries9–12. 1234567890():,; 12 Predicting aqueous stability of solids is of central importance to Specifically, Zhang et al. demonstrated that the formation materials development for electrochemical applications such as energies of 196 binary compounds using the SCAN functional fuel cells, electrolyzers, and Li-air batteries1–4. The electrode reduce the mean absolute error (MAE) to 0.1 eV/atom, halving that potential-pH (aka. Pourbaix) diagram maps the most stable of the PBE functional. -

Development Team
Paper No: 16 Environmental Chemistry Module: 23 Aquatic Redox Chemistry Development Team Prof. R.K. Kohli Principal Investigator & Prof. V.K. Garg & Prof. Ashok Dhawan Co- Principal Investigator Central University of Punjab, Bathinda Prof. K.S. Gupta Paper Coordinator University of Rajasthan, Jaipur Dr. Alka Sharma Content Writer University of Rajasthan, Jaipur Content Reviewer Prof. K.S. Gupta University of Rajasthan, Jaipur Anchor Institute Central University of Punjab 1 Environmental Chemistry Environmental Sciences Aquatic Redox Chemistry Description of Module Subject Name Environmental Sciences Paper Name Environmental Chemistry Module Name/Title Aquatic Redox Chemistry Module Id EVS/EC-XVI/23 Pre-requisites A basic knowledge of redox chemistry 1. To define redox (e- transfer) reactions, half reactions 2. To define and understand aquatic redox chemistry 3. To define redox potential, cell reactions and cell potential 4. To define standard electrode potential E0 and Nernst equation Objectives 5. To understand pE-scale, significance and measurement of pE values 6. To understand redox ladder 7. To understand redox potential in natural water, its interpretation and significance 8. To define and understand applications of redox potentials in aquatic systems Redox reactions, redox process, redox potential, pE-scale, redox ladder, aquatic redox Keywords system 2 Environmental Chemistry Environmental Sciences Aquatic Redox Chemistry Module 23: Aquatic Redox Chemistry Contents 1. Introduction 2. Half Reactions 3. Reduction Potential 4. Standard Reduction Potential 5. Interpretation and Significance 6. Cell Reaction and Cell Potential 7. Hydrogen Electrode and determination of Electrode Potential 8. pE-Scale 9. Significance of pE Values 10. Measurement of pE-Values 11. Solved Problem 12. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Theoretical and Experimental Investigation of the Effects and Limits of using Inorganic Aqueous Solutions to Resist NCG Generation in Aluminum Thermosiphons Permalink https://escholarship.org/uc/item/4nk859pk Author Stubblebine, Michael James Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Theoretical and Experimental Investigation of the Effects and Limits of using Inorganic Aqueous Solutions to Resist NCG Generation in Aluminum Thermosiphons A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Mechanical Engineering by Michael James Stubblebine 2016 © Copyright by Michael James Stubblebine 2016 ABSTRACT OF THE DISSERTATION Theoretical and Experimental Investigation of the Effects and Limits of using Inorganic Aqueous Solutions to Resist NCG Generation in Aluminum Thermosiphons by Michael James Stubblebine Doctor of Philosophy in Mechanical Engineering University of California, Los Angeles, 2016 Professor Ivan Catton, Chair Water is one of the most capable and widely used working fluids in heat pipes and thermosiphons. Aluminum is a high conductivity, lightweight, and high strength choice for heat pipe casing material. However, aluminum and water are a heat pipe combination that is not considered viable due to the rapid generation of hydrogen, a noncondensable gas (NCG), resulting from chemical reactions between water and aluminum. A great deal of past research has been done evaluating the compatibility of many pure fluids with different metal heat pipe casings, yielding a large volume of lifetime tests demonstrating success or failure of various combinations. On the other hand, very little research was found analyzing what, if any, progress can be made to take low compatibility combinations and make them more compatible through the use of chemical ii inhibitors. -

O Simplified Procedure for Constructing Pourbaix Diagrams*
O Simplified Procedure for Constructing Pourbaix Diagrams* E. D. VERINK, JR. Distinguished Service Professor Emeritus Department of Materials Science and Engineering University of Florida Gainesville, Florida A. INTRODUCTION For the behavior of metals in aqueous solutions, one of the most important contributions to the corrosion literature has been the work of Pourbaix [1,2] and his associates in the development of thermodynamic equilibrium diagrams (E vs. pH), called Pourbaix diagrams. A vast amount of data may be presented simply and concisely in Pourbaix diagrams. When the advantages and limitations of such diagrams are understood, valuable inferences may be made regarding corrosion phenomena. The selection of conditions for cathodic and anodic protection is simplified. Candidates for consi- deration as inhibitor species may be selected with greater efficiency. Critical corrosion experiments may be designed with equal efficiency. Corrosion processes involve both chemical and electrochemical phenomena. In 1923, Evans [3] observed that, if two samples of iron connected by a galvanometer are immersed in two solutions of potassium chloride, separated by a porous membrane, and if a stream of air is bubbled through one of these solutions, an electric current circulates between the aerated sample, which becomes the cathode, and the nonaerated sample, which becomes the anode and corrodes. On the other hand, if a sample of iron and a sample of another metal (copper, zinc, or magnesium) are connected as above, a passage of electric current is also observed. Under these circumstances, iron becomes the anode and corrodes when connected to copper, whereas zinc or magnesium become the anode and corrode providing protection to iron.