Mechanisms of Sensory Working Memory: Attention and Perfomance
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Learning Styles and Memory
Learning Styles and Memory Sandra E. Davis Auburn University Abstract The purpose of this article is to examine the relationship between learning styles and memory. Two learning styles were addressed in order to increase the understanding of learning styles and how they are applied to the individual. Specifically, memory phases and layers of memory will also be discussed. In conclusion, an increased understanding of the relationship between learning styles and memory seems to help the learner gain a better understanding of how to the maximize benefits for the preferred leaning style and how to retain the information in long-term memory. Introduction Learning styles, as identified in the Perpetual Learning Styles Theory and memory, as identified in the Memletics Accelerated Learning, will be overviewed. Factors involving information being retained into memory will then be discussed. This article will explain how the relationship between learning styles and memory can help the learner maximize his or her learning potential. Learning Styles The Perceptual Learning Styles Theory lists seven different styles. They are print, aural, interactive, visual, haptic, kinesthetic, and olfactory (Institute for Learning Styles Research, 2003). This theory says that most of what we learn comes from our five senses. The Perceptual Learning Style Theory defines the seven learning styles as follows: The print learning style individual prefers to see the written word (Institute for Learning Styles Research, 2003). They like taking notes, reading books, and seeing the written word, either on a chalk board or thru a media presentation such as Microsoft Powerpoint. The aural learner refers to listening (Institute for Learning Styles Research, 2003). -

High 1 Effectiveness of Echoic and Iconic Memory in Short-Term and Long-Term Recall Courtney N. High 01/14/13 Mr. Mengel Psychol
High 1 Effectiveness of Echoic and Iconic Memory in Short-term and Long-term Recall Courtney N. High 01/14/13 Mr. Mengel Psychology 1 High 2 Abstract Objective: To see whether iconic memory or echoic memory is more effective at being stored and recalled as short-term and long-term memory in healthy adults. Method: Eight healthy adults between the ages of 18 and 45 were tested in the study. Participants were shown a video containing ten pictures and ten sounds of easily recognizable objects. Participants were asked to recall as many items as they could immediately after the video and were then asked again after a series of questions. Results: In younger adults more visual objects are able to be recalled both short and long term, but with older adults, in short term recall, the same number of sound and visual items where remembered, and with long term recall, sound items were remembered slightly better. Results also showed that iconic memory fades faster than echoic memory. Conclusion: The ability to store and recall iconic and echoic information both short and long term varies with age. The study has several faults including relying on self-reporting on health for participants, and testing environments not being quiet in all tests. Introduction There are three main different types of memory: Sensory memory, short-term memory, and long-term memory. Sensory memory deals with the brief storage of information immediately after stimulation. Sensory memory is then converted to short-term memory if deemed necessary by the brain where it is held. After that, some information will then be stored as long-term memory for later recall. -

Cognitive Functions of the Brain: Perception, Attention and Memory
IFM LAB TUTORIAL SERIES # 6, COPYRIGHT c IFM LAB Cognitive Functions of the Brain: Perception, Attention and Memory Jiawei Zhang [email protected] Founder and Director Information Fusion and Mining Laboratory (First Version: May 2019; Revision: May 2019.) Abstract This is a follow-up tutorial article of [17] and [16], in this paper, we will introduce several important cognitive functions of the brain. Brain cognitive functions are the mental processes that allow us to receive, select, store, transform, develop, and recover information that we've received from external stimuli. This process allows us to understand and to relate to the world more effectively. Cognitive functions are brain-based skills we need to carry out any task from the simplest to the most complex. They are related with the mechanisms of how we learn, remember, problem-solve, and pay attention, etc. To be more specific, in this paper, we will talk about the perception, attention and memory functions of the human brain. Several other brain cognitive functions, e.g., arousal, decision making, natural language, motor coordination, planning, problem solving and thinking, will be added to this paper in the later versions, respectively. Many of the materials used in this paper are from wikipedia and several other neuroscience introductory articles, which will be properly cited in this paper. This is the last of the three tutorial articles about the brain. The readers are suggested to read this paper after the previous two tutorial articles on brain structure and functions [17] as well as the brain basic neural units [16]. Keywords: The Brain; Cognitive Function; Consciousness; Attention; Learning; Memory Contents 1 Introduction 2 2 Perception 3 2.1 Detailed Process of Perception . -

Role of Sleep in Memory 1Racheal Fernandes, 2Preeti Devnani
IJSM Racheal Fernandes, Preeti Devnani 10.5005/jp-journals-10069-0003 REVIEW ARTICLE Role of Sleep in Memory 1Racheal Fernandes, 2Preeti Devnani ABSTRACT on the specific conditions of learning and the timing of Sleep is essential to our survival. The various stages of sleep sleep periods. play a significant role in brain maturation. Memory is an important There are two broad types of memory: Short memory essential feature of the human brain. Memory is information that is and long-term memory. It is generally assumed that there encoded, retained, and recalled. This review summarizes various are two kinds of memory: Natural memory, those that are subtypes of memory and provides insight into the role of sleep inborn memory that are required to live our day-to-day in the formation of memory and the impact of sleep deprivation. life, and artificial memory, those that are acquired during Keywords: Memory formation, Sleep deprivation and memory. the course of our lives. Memory is essential to behavior, How to cite this article: Fernandes R, Devnani P. Role of Sleep enabling organisms to draw on past experience to guide in Memory. Indian Sleep Med 2017;12(2):12-14. choices and actions. Source of support: Nil Conflict of interest: None TYPES OF MEMORY The human memory is divided into sensory memory, INTRODUCTION short-term memory (working memory), and long-term memory (Flow Chart 1), each of which has a particular Sleep is not just “time out” from daily life. Sleep is an mode of operation and is involved in the process of active dynamic process and is important for renewing memorization. -

Cognitive Psychology Overview
By the Numbers http://ipt.byu.edu/isee/admin/print/index.php?story=2 Cognitive Psychology Overview How does memory affect learning? What types of information are more difficult to remember? What strategies are useful for storing and retrieving information? How can a teacher facilitate learning by better understanding memory? The purpose of this chapter is to help you learn about the Information Processing Model and other related cognitive concepts through encountering and resolving a classroom problem. The problem this story is based on was a regular concern with one of the authors' 3rd-grade classes, and one you're likely to encounter as a teacher. Cognitive psychology (also known as cognitivism), a term used to describe "the process of thought," is a branch of educational psychology that explores internal mental processes (Neisser, 1967). There are 6 different thought processes in cognitive psychology that have been researched extensively. 1. perception 2. attention 3. memory 4. problem solving 5. reasoning 6. decision making PAMPRD is a mnemonic to help you remember these 6 mental processes of cognition. They combine to influence behavior and learning. These will be discussed in greater detail in this chapter. Various models have been developed by cognitive theorists to describe how individuals think and process information. The most commonly accepted model of how the brain structures memory is referred to as the Information Processing Model (Atkinson & Shiffrin, 1968), which addresses perception, attention, and memory (PAM) in the six thought processes described above. Cognitive theories propose that the mind has a general architecture for processing information, often comparing the way our minds process information to the workings of a computer. -

Okami Study Guide: Chapter 8 1
Okami Study Guide: Chapter 8 1 Chapter in Review 1. Memory may be defined as a group of mechanisms and systems that encode, store, and retrieve information. The modal model of memory describes three stages and stores in the memory process: sensory memory, short-term memory (STM), and long- term memory (LTM). 2. Sensory memory very briefly stores fleeing sensory impressions for further processing in STM and LTM. Sensory memory is divided into two categories: iconic store, which stores fleeting visual impressions; and echoic store, which stores fleeting auditory impressions. In addition to storing sensory impressions for further processing, sensory memory allows us to perceive the world as a continuous stream of events instead of a series of “snapshots.” 3. When you consciously or unconsciously decide to pay attention to specific pieces of information in sensory memory, the information is transferred into short-term memory. The duration and capacity of STM are limited. In general, information can remain in STM for no longer than 20 seconds unless maintenance rehearsal takes place, and no more than 4 single items or chunks of information can be held in STM at any one time. A chunk is any grouping of items that are strongly associated with one another. 4. Long-term memory (LTM) is theoretically limitless and relatively permanent. Information moves from STM to LTM when it is encoded in one of three ways: through sound (acoustic encoding), imagery (visual encoding), or meaning (semantic encoding). Encoding in STM tends to be primarily acoustic, secondarily visual, and much less often semantic. However, encoding in LTM is most effective if it is semantic. -
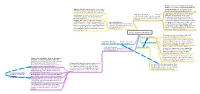
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Memory: Issues of Import to School Psychologists
DOCUMENT RESUME ED 365 927 CG 025 192 AUTHOR John, Kirk R. TITLE Memory: Issues of Import to School Psychologists PUB DATE Apr 93 NOTE 24p.; Paper presented at the Annual Meeting of the National Association of School Psychologists (25th, Washington, DC, April 13-17, 1993). PUB TYPE Speeches/Conference Papers (150) Guides General (050) EDRS PRICE MF01/PC01 Plus Postage. DESCRIPTORS Adolescent Development; Adolescents; Child Development; Children; *Cognitive Development; Elementary Secondary Education; *Memory; *School Psychologists ABSTRACT This document defines memory as a complex, interactive process that is a prerequisite for all higher learning. Without intact memory skills, a host of disorders may ensue ranging frow mild learning problems to disorientation and helplessness (Lezak, 1983). Because of the pervasive and central role memory plays in people's lives, school psychologists should have at the very least a basic understanding of the memory process. In this regard, this paper addresses selected topics from the knowledge base on memory that have relevance for school psychologists. First, an information-processing model of memory is presented and the three separate memory systems through which information is processed are described (sensory memory, short-term memory, and long-term memory). A section on the developmental aspects of memory considers memory development in infants, children and adolescents. Metamemory, or the individual's conscious awareness of his/her own memory capabilities and functions, also is explained in this section. The final major section of the paper focuses on memory and reading. This information is discussed in the context of how it can be applied by school psychologists in their decision-making. -

The Aging Brain and Neuroplasticity
POWER OF POSITIVE AGING NEUROPLASTICITY AND THE AGING BRAIN Worlds First Senior Moment . QUIZ • Loss of memory is a normal part of aging • The brain can form new connections throughout life • We can predict which individuals with memory complaints will progress to Alzheimer’s disease dementia THERE WILL BE A LOT OF AGING BRAINS • By 2029, 20% of population over 65 • 65 y/o will live, on average, 20 more years • Effect of 10-25% reduction in 7 risk factors may prevent up to 500,000 cases of AD IS MEMORY LOSS A NORMAL PART OF AGING MEMORY • Multiple sub-systems: Episodic (personally relevant events/episodes) Working (manipulate learned info) Semantic (knowledge of facts, meaning of words) Procedural (performance of skills) • Can run independently MEMORY PROCESS Sensations Sensations Sensations Sensations Immediate Memory 1. encoding Short Term Memory 3. Retrieval 2. storage Long Term Memory WHAT’S NORMAL, WHAT’S NOT? Normal Aging Cognitive Function Years NORMAL COGNITIVE AGING • Slowing of central processing • Mild changes in memory • Other cognitive abilities relatively preserved NORMAL AGING: COGNITIVE CHANGES 50 YO +20% 80 YO 20 YO -20% -40% -60% Vocabulary Mental Verbal Fluency Math NORMAL AGING: COGNITIVE CHANGES 50 YO +20% 80 YO 20 YO -20% -40% -60% Selective Mental Visual- Attention Flexibility Construction AGE-RELATED MEMORY/COGNITIVE CHANGES • Few changes: • Declines: • Crystallized Intelligence • Sensory Memory • Procedural Memory • Short-term Memory • Complex/Selective Attention • Long-term Memory • Executive Skills • Auditory -

Memory and Cognition
947 Memory and Cognition MEMORY AND COGNITION Your knowledge in this Neuroscience course should be closely related to the Neurological Exam. In the “Mini Mental” part this exam you will ask the patient if you can test their memory. To do this, state the name of three unrelated items (dog, pencil, ball) and then ask the patient to repeat the three items. This is testing their “short term or working memory”. Then ask the patient to remember these three items, because you will ask him/her to repeat them 3-5 minutes later. Make certain all three objects have been registered and provide distracters during the delay period to prevent the patient from rehearsing the items repeatedly. Then, (after 3-5 minutes), ask your patient to recall the three unrelated items. This is testing what is called “recent memory”. Finally, you will test your patient for what is called “remote memory”. This is done by asking the patient about historical or verifiable personal events of the past. The following overview should help you und understand the biology underlying these different types of memory tests. PLEASE read this with interest and enthusiasm and realize that what you will be tested on relates to what you will use as a physician! The Practice Questions are 100% indicative of the level of understanding expected of you. Memory is a very interesting yet still poorly understood aspect of cognition. AN OVERVIEW OF MEMORY Sensory Memory All incoming information is held briefly (1/2 to 2 seconds) in sensory memory as a copy of the actual sensory information (for example, visual stimuli will be held briefly in visual cortex/area 17) . -

Characterizing the Neuro-Cognitive Architecture of Non-Conscious Working Memory Darinka Trübutschek
Characterizing the neuro-cognitive architecture of non-conscious working memory Darinka Trübutschek To cite this version: Darinka Trübutschek. Characterizing the neuro-cognitive architecture of non-conscious working mem- ory. Cognitive Sciences. Sorbonne Université, 2018. English. NNT : 2018SORUS101. tel-02956592 HAL Id: tel-02956592 https://tel.archives-ouvertes.fr/tel-02956592 Submitted on 3 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université Pierre et Marie Curie Ecole doctorale Cerveau, Cognition et Comportement Inserm-CEA Cognitive Neuroimaging Unit (Unicog, Neurospin) CHARACTERIZING THE NEURO-COGNITIVE ARCHITECTURE OF NON-CONSCIOUS WORKING MEMORY Darinka TRÜBUTSCHEK Dissertation submitted in partial fulfillment for the degree of Doctor in Philosophy (PhD) in Cognitive Neuroscience Advised by Prof. Stanislas DEHAENE and co-advised by Dr. Sébastien MARTI Presented and publically defended on the 1st of October 2018 In front of a jury and invited members composed of: Prof. David SOTO Basque Center on Cognition, Brain, and Language Reviewer Prof. Mark STOKES University of Oxford Reviewer Prof. Paolo BARTOLOMEO Institut du Cerveau et de la Moelle épinière Examiner Dr. Lucie CHARLES University College London Examiner Prof. Stanislas DEHAENE Neurospin Examiner Dr. Sébastien MARTI Neurospin Examiner Prof. -

4.4 TYPES of MEMORY the Last Two Sections Focused on Nature of Memory and the Various Models of Memory
4.1 INTRODUCTION Memory Consider the following examples: Do you know how to ride a bike? If you know, then how much do you think about rotating the pedals or balancing while riding the bike? How much time do you take while processing the information of a repeated television advertisement that you are watching? Do you remember the last time you met your school teacher? Instances mentioned above and other such instances, highlight the importance of memory in everyday life. The term memory refers to conscious retrieval of previously experienced information. So, for the above instances, the process of conscious retrieval of the experienced information is the part of the process. However, all instances involve different types of memory! A glance over the two preceding units on Perception and Learning, reflect that the two processes are important for human behavior along with Memory. Perception, learning and memory are closely linked. An object or event is perceived, learned, memorized and recalled, thereby helping the individual to adapt. In this unit, we shall turn our attention to the process of memory. In the first part of the unit, nature, scope and models of memory will be explained followed by types of memory. In the latter part of the unit, we shall summarize about the process of forgetting and the strategies to improve memory. 4.2 NATURE AND SCOPE OF MEMORY What did you eat in dinner yesterday? What is the name of your best friend? Do you know how to drive a car or ride a cycle? How did you feel when you got highest marks in your class? The mental process you used to answer all of these questions is known as memory.