Characterizing the Neuro-Cognitive Architecture of Non-Conscious Working Memory Darinka Trübutschek
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Memory in Mind and Culture
This page intentionally left blank Memory in Mind and Culture This text introduces students, scholars, and interested educated readers to the issues of human memory broadly considered, encompassing individual mem- ory, collective remembering by societies, and the construction of history. The book is organized around several major questions: How do memories construct our past? How do we build shared collective memories? How does memory shape history? This volume presents a special perspective, emphasizing the role of memory processes in the construction of self-identity, of shared cultural norms and concepts, and of historical awareness. Although the results are fairly new and the techniques suitably modern, the vision itself is of course related to the work of such precursors as Frederic Bartlett and Aleksandr Luria, who in very different ways represent the starting point of a serious psychology of human culture. Pascal Boyer is Henry Luce Professor of Individual and Collective Memory, departments of psychology and anthropology, at Washington University in St. Louis. He studied philosophy and anthropology at the universities of Paris and Cambridge, where he did his graduate work with Professor Jack Goody, on memory constraints on the transmission of oral literature. He has done anthro- pological fieldwork in Cameroon on the transmission of the Fang oral epics and on Fang traditional religion. Since then, he has worked mostly on the experi- mental study of cognitive capacities underlying cultural transmission. After teaching in Cambridge, San Diego, Lyon, and Santa Barbara, Boyer moved to his present position at the departments of anthropology and psychology at Washington University, St. Louis. James V. -

Learning Styles and Memory
Learning Styles and Memory Sandra E. Davis Auburn University Abstract The purpose of this article is to examine the relationship between learning styles and memory. Two learning styles were addressed in order to increase the understanding of learning styles and how they are applied to the individual. Specifically, memory phases and layers of memory will also be discussed. In conclusion, an increased understanding of the relationship between learning styles and memory seems to help the learner gain a better understanding of how to the maximize benefits for the preferred leaning style and how to retain the information in long-term memory. Introduction Learning styles, as identified in the Perpetual Learning Styles Theory and memory, as identified in the Memletics Accelerated Learning, will be overviewed. Factors involving information being retained into memory will then be discussed. This article will explain how the relationship between learning styles and memory can help the learner maximize his or her learning potential. Learning Styles The Perceptual Learning Styles Theory lists seven different styles. They are print, aural, interactive, visual, haptic, kinesthetic, and olfactory (Institute for Learning Styles Research, 2003). This theory says that most of what we learn comes from our five senses. The Perceptual Learning Style Theory defines the seven learning styles as follows: The print learning style individual prefers to see the written word (Institute for Learning Styles Research, 2003). They like taking notes, reading books, and seeing the written word, either on a chalk board or thru a media presentation such as Microsoft Powerpoint. The aural learner refers to listening (Institute for Learning Styles Research, 2003). -

High 1 Effectiveness of Echoic and Iconic Memory in Short-Term and Long-Term Recall Courtney N. High 01/14/13 Mr. Mengel Psychol
High 1 Effectiveness of Echoic and Iconic Memory in Short-term and Long-term Recall Courtney N. High 01/14/13 Mr. Mengel Psychology 1 High 2 Abstract Objective: To see whether iconic memory or echoic memory is more effective at being stored and recalled as short-term and long-term memory in healthy adults. Method: Eight healthy adults between the ages of 18 and 45 were tested in the study. Participants were shown a video containing ten pictures and ten sounds of easily recognizable objects. Participants were asked to recall as many items as they could immediately after the video and were then asked again after a series of questions. Results: In younger adults more visual objects are able to be recalled both short and long term, but with older adults, in short term recall, the same number of sound and visual items where remembered, and with long term recall, sound items were remembered slightly better. Results also showed that iconic memory fades faster than echoic memory. Conclusion: The ability to store and recall iconic and echoic information both short and long term varies with age. The study has several faults including relying on self-reporting on health for participants, and testing environments not being quiet in all tests. Introduction There are three main different types of memory: Sensory memory, short-term memory, and long-term memory. Sensory memory deals with the brief storage of information immediately after stimulation. Sensory memory is then converted to short-term memory if deemed necessary by the brain where it is held. After that, some information will then be stored as long-term memory for later recall. -

2.33 Implicit Memory and Priming W
2.33 Implicit Memory and Priming W. D. Stevens, G. S. Wig, and D. L. Schacter, Harvard University, Cambridge, MA, USA ª 2008 Elsevier Ltd. All rights reserved. 2.33.1 Introduction 623 2.33.2 Influences of Explicit Versus Implicit Memory 624 2.33.3 Top-Down Attentional Effects on Priming 626 2.33.3.1 Priming: Automatic/Independent of Attention? 626 2.33.3.2 Priming: Modulated by Attention 627 2.33.3.3 Neural Mechanisms of Top-Down Attentional Modulation 629 2.33.4 Specificity of Priming 630 2.33.4.1 Stimulus Specificity 630 2.33.4.2 Associative Specificity 632 2.33.4.3 Response Specificity 632 2.33.5 Priming-Related Increases in Neural Activation 634 2.33.5.1 Negative Priming 634 2.33.5.2 Familiar Versus Unfamiliar Stimuli 635 2.33.5.3 Sensitivity Versus Bias 636 2.33.6 Correlations between Behavioral and Neural Priming 637 2.33.7 Summary and Conclusions 640 References 641 2.33.1 Introduction stimulus that result from a previous encounter with the same or a related stimulus. Priming refers to an improvement or change in the When considering the link between behavioral identification, production, or classification of a stim- and neural priming, it is important to acknowledge ulus as a result of a prior encounter with the same or that functional neuroimaging relies on a number of a related stimulus (Tulving and Schacter, 1990). underlying assumptions. First, changes in informa- Cognitive and neuropsychological evidence indi- tion processing result in changes in neural activity cates that priming reflects the operation of implicit within brain regions subserving these processing or nonconscious processes that can be dissociated operations. -

Associative Repetition Priming: a Selective Review and Theoretical Implications
Associative repetition priming 1 Running head: Associative Repetition Priming Associative repetition priming: A selective review and theoretical implications René Zeelenberg University of Amsterdam Diane Pecher Utrecht University and Jeroen G.W. Raaijmakers University of Amsterdam Send correspondence to: René Zeelenberg Psychology Department Associative repetition priming 2 Indiana University Bloomington, IN 47405 USA phone: 001-812-856-5217 fax: 001-812-855-1086 email: [email protected] Associative repetition priming 3 A. Introduction An extensive body of research in the last two decades has shown that recent experiences can affect performance even when subjects are not instructed to remember these earlier experiences and even when subjects are not aware of these experiences. These so-called implicit memory phenomena are usually demonstrated by the repetition priming effect. Repetition priming refers to the finding that responses are faster and more accurate to stimuli that have been encountered recently than to stimuli that have not been encountered recently. For example, in a perceptual word identification task (Jacoby & Dallas, 1981; Salasoo, Shiffrin & Feustel, 1985) subjects can more often correctly identify the briefly flashed target word if they have recently studied the target than if they have not studied the target. Comparable effects have been obtained in tasks such as picture identification (e.g., Rouder, Ratcliff & McKoon, 2000), word stem completion (e.g., Graf, Squire, & Mandler, 1984) and lexical decision (e.g., Bowers & Michita, 1998; Scarborough, Cortese, & Scarborough, 1977), to name but a few. The large majority of studies in the domain of implicit memory have concentrated on the effect of repeating stimuli in isolation. In the common repetition priming paradigm stimuli are presented one-by-one both during the study phase and the test phase. -

Cognitive Functions of the Brain: Perception, Attention and Memory
IFM LAB TUTORIAL SERIES # 6, COPYRIGHT c IFM LAB Cognitive Functions of the Brain: Perception, Attention and Memory Jiawei Zhang [email protected] Founder and Director Information Fusion and Mining Laboratory (First Version: May 2019; Revision: May 2019.) Abstract This is a follow-up tutorial article of [17] and [16], in this paper, we will introduce several important cognitive functions of the brain. Brain cognitive functions are the mental processes that allow us to receive, select, store, transform, develop, and recover information that we've received from external stimuli. This process allows us to understand and to relate to the world more effectively. Cognitive functions are brain-based skills we need to carry out any task from the simplest to the most complex. They are related with the mechanisms of how we learn, remember, problem-solve, and pay attention, etc. To be more specific, in this paper, we will talk about the perception, attention and memory functions of the human brain. Several other brain cognitive functions, e.g., arousal, decision making, natural language, motor coordination, planning, problem solving and thinking, will be added to this paper in the later versions, respectively. Many of the materials used in this paper are from wikipedia and several other neuroscience introductory articles, which will be properly cited in this paper. This is the last of the three tutorial articles about the brain. The readers are suggested to read this paper after the previous two tutorial articles on brain structure and functions [17] as well as the brain basic neural units [16]. Keywords: The Brain; Cognitive Function; Consciousness; Attention; Learning; Memory Contents 1 Introduction 2 2 Perception 3 2.1 Detailed Process of Perception . -

Role of Sleep in Memory 1Racheal Fernandes, 2Preeti Devnani
IJSM Racheal Fernandes, Preeti Devnani 10.5005/jp-journals-10069-0003 REVIEW ARTICLE Role of Sleep in Memory 1Racheal Fernandes, 2Preeti Devnani ABSTRACT on the specific conditions of learning and the timing of Sleep is essential to our survival. The various stages of sleep sleep periods. play a significant role in brain maturation. Memory is an important There are two broad types of memory: Short memory essential feature of the human brain. Memory is information that is and long-term memory. It is generally assumed that there encoded, retained, and recalled. This review summarizes various are two kinds of memory: Natural memory, those that are subtypes of memory and provides insight into the role of sleep inborn memory that are required to live our day-to-day in the formation of memory and the impact of sleep deprivation. life, and artificial memory, those that are acquired during Keywords: Memory formation, Sleep deprivation and memory. the course of our lives. Memory is essential to behavior, How to cite this article: Fernandes R, Devnani P. Role of Sleep enabling organisms to draw on past experience to guide in Memory. Indian Sleep Med 2017;12(2):12-14. choices and actions. Source of support: Nil Conflict of interest: None TYPES OF MEMORY The human memory is divided into sensory memory, INTRODUCTION short-term memory (working memory), and long-term memory (Flow Chart 1), each of which has a particular Sleep is not just “time out” from daily life. Sleep is an mode of operation and is involved in the process of active dynamic process and is important for renewing memorization. -

Cognitive Psychology Overview
By the Numbers http://ipt.byu.edu/isee/admin/print/index.php?story=2 Cognitive Psychology Overview How does memory affect learning? What types of information are more difficult to remember? What strategies are useful for storing and retrieving information? How can a teacher facilitate learning by better understanding memory? The purpose of this chapter is to help you learn about the Information Processing Model and other related cognitive concepts through encountering and resolving a classroom problem. The problem this story is based on was a regular concern with one of the authors' 3rd-grade classes, and one you're likely to encounter as a teacher. Cognitive psychology (also known as cognitivism), a term used to describe "the process of thought," is a branch of educational psychology that explores internal mental processes (Neisser, 1967). There are 6 different thought processes in cognitive psychology that have been researched extensively. 1. perception 2. attention 3. memory 4. problem solving 5. reasoning 6. decision making PAMPRD is a mnemonic to help you remember these 6 mental processes of cognition. They combine to influence behavior and learning. These will be discussed in greater detail in this chapter. Various models have been developed by cognitive theorists to describe how individuals think and process information. The most commonly accepted model of how the brain structures memory is referred to as the Information Processing Model (Atkinson & Shiffrin, 1968), which addresses perception, attention, and memory (PAM) in the six thought processes described above. Cognitive theories propose that the mind has a general architecture for processing information, often comparing the way our minds process information to the workings of a computer. -

Okami Study Guide: Chapter 8 1
Okami Study Guide: Chapter 8 1 Chapter in Review 1. Memory may be defined as a group of mechanisms and systems that encode, store, and retrieve information. The modal model of memory describes three stages and stores in the memory process: sensory memory, short-term memory (STM), and long- term memory (LTM). 2. Sensory memory very briefly stores fleeing sensory impressions for further processing in STM and LTM. Sensory memory is divided into two categories: iconic store, which stores fleeting visual impressions; and echoic store, which stores fleeting auditory impressions. In addition to storing sensory impressions for further processing, sensory memory allows us to perceive the world as a continuous stream of events instead of a series of “snapshots.” 3. When you consciously or unconsciously decide to pay attention to specific pieces of information in sensory memory, the information is transferred into short-term memory. The duration and capacity of STM are limited. In general, information can remain in STM for no longer than 20 seconds unless maintenance rehearsal takes place, and no more than 4 single items or chunks of information can be held in STM at any one time. A chunk is any grouping of items that are strongly associated with one another. 4. Long-term memory (LTM) is theoretically limitless and relatively permanent. Information moves from STM to LTM when it is encoded in one of three ways: through sound (acoustic encoding), imagery (visual encoding), or meaning (semantic encoding). Encoding in STM tends to be primarily acoustic, secondarily visual, and much less often semantic. However, encoding in LTM is most effective if it is semantic. -
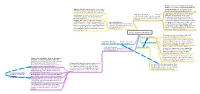
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Memory: Issues of Import to School Psychologists
DOCUMENT RESUME ED 365 927 CG 025 192 AUTHOR John, Kirk R. TITLE Memory: Issues of Import to School Psychologists PUB DATE Apr 93 NOTE 24p.; Paper presented at the Annual Meeting of the National Association of School Psychologists (25th, Washington, DC, April 13-17, 1993). PUB TYPE Speeches/Conference Papers (150) Guides General (050) EDRS PRICE MF01/PC01 Plus Postage. DESCRIPTORS Adolescent Development; Adolescents; Child Development; Children; *Cognitive Development; Elementary Secondary Education; *Memory; *School Psychologists ABSTRACT This document defines memory as a complex, interactive process that is a prerequisite for all higher learning. Without intact memory skills, a host of disorders may ensue ranging frow mild learning problems to disorientation and helplessness (Lezak, 1983). Because of the pervasive and central role memory plays in people's lives, school psychologists should have at the very least a basic understanding of the memory process. In this regard, this paper addresses selected topics from the knowledge base on memory that have relevance for school psychologists. First, an information-processing model of memory is presented and the three separate memory systems through which information is processed are described (sensory memory, short-term memory, and long-term memory). A section on the developmental aspects of memory considers memory development in infants, children and adolescents. Metamemory, or the individual's conscious awareness of his/her own memory capabilities and functions, also is explained in this section. The final major section of the paper focuses on memory and reading. This information is discussed in the context of how it can be applied by school psychologists in their decision-making. -

Repetition Priming Mediated by Task Similarity in Semantic Classification
Memory & Cognition 2003, 31 (7), 1009-1020 Repetition priming mediated by task similarity in semantic classification MAGGIE J. XIONG, JEFFERY J. FRANKS, and GORDON D. LOGAN Vanderbilt University, Nashville, Tennessee In the present study, the specificityof repetition priming between semantic classificationtasks was ex- amined using Osgood’s (Osgood, Suci, & Tannenbaum, 1957) semantic space as a heuristic for deter- mining the similarity between classifications.The classificationtasks involved judging the meaning of words on semantic scales,such as pleasant/unpleasant. The amount of priming across classifications was hypothesized to decreasewith increasing distance (decreasing similarity)between semantic scales in connotative semantic space. The results showed maximum repetition priming when the study and the test classificationswere the same, intermediate degrees of priming when the study and the test classi- fication scales shared loadings on semantic factors, and little priming when the study and the test clas- sification scales loaded primarily on orthogonal semantic factors—that is, when the distance between scales was maximized. Consistent with the transfer-appropriateprocessing framework, repetition prim- ing in semantic classifications was highly task specific, decreasing with increasing distance between classificationscales. Repetition priming is a facilitation or a bias in perfor- In the present study, the specificity of repetition mance on a stimulus that results from past experience with priming—that is, the specificity of transfer between se- the stimulus. It manifests experimentallyas faster reaction mantic classification tasks—was examined. From a strict time (RT), improved accuracy, or bias toward one response TAP perspective, repetition priming should be highly task over another (Jacoby & Dallas, 1981; Jacoby & Wither- specific and sensitive to the similarity between study and spoon, 1982; McAndrews & Moscovitch,1990; Ratcliff & test tasks.