Ischemic Disease of the Intestine P.H
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Inflammatory Bowel Disease Irritable Bowel Syndrome
Inflammatory Bowel Disease and Irritable Bowel Syndrome Similarities and Differences 2 www.ccfa.org IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 Important Differences Between IBD and IBS Many diseases and conditions can affect the gastrointestinal (GI) tract, which is part of the digestive system and includes the esophagus, stomach, small intestine and large intestine. These diseases and conditions include inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 www.ccfa.org 3 Inflammatory bowel diseases are a group of inflammatory conditions in which the body’s own immune system attacks parts of the digestive system. Inflammatory Bowel Disease Inflammatory bowel diseases are a group of inflamma- Causes tory conditions in which the body’s own immune system attacks parts of the digestive system. The two most com- The exact cause of IBD remains unknown. Researchers mon inflammatory bowel diseases are Crohn’s disease believe that a combination of four factors lead to IBD: a (CD) and ulcerative colitis (UC). IBD affects as many as 1.4 genetic component, an environmental trigger, an imbal- million Americans, most of whom are diagnosed before ance of intestinal bacteria and an inappropriate reaction age 35. There is no cure for IBD but there are treatments to from the immune system. Immune cells normally protect reduce and control the symptoms of the disease. the body from infection, but in people with IBD, the immune system mistakes harmless substances in the CD and UC cause chronic inflammation of the GI tract. CD intestine for foreign substances and launches an attack, can affect any part of the GI tract, but frequently affects the resulting in inflammation. -

A Case Report of Fibro-Stenotic Crohn's Disease in the Middle
DOI: https://doi.org/10.22516/25007440.185 Case report A Case Report of Fibro-Stenotic Crohn’s Disease in the Middle Ileum as the Initial Manifestation Adriana Margarita Rey,1 Gustavo Reyes,1 Fernando Sierra,1 Rafael García-Duperly,2 Rocío López,3 Leidy Paola Prada.4 1 Gastroenterologist in the Gastroenterology and Abstract Hepatology Service of the Hospital Universitario Fundación Santa Fe de Bogotá in Bogotá, Colombia Crohn’s disease (CD) is an inflammatory bowel disease that can affect the entire gastrointestinal tract. The small 2 Colon and Rectum Surgeon in the Department of intestine is affected in about 50% of patients among whom the terminal ileum is the area most commonly affected. Surgery of the Hospital Universitario Fundación Intestinal stenosis is a common complication in CD and approximately 30% to 50% of patients present Santa Fe de Bogotá in Bogotá, Colombia 3 Pathologist at of the Hospital Universitario Fundación stenosis or penetrating lesions at the time of diagnosis. Because conventional endoscopic techniques do not Santa Fe de Bogotá and Professor at Universidad de allow evaluation of small bowel lesions, techniques such as enteroscopy and endoscopic video-capsule were los Andes in Bogotá, Colombia developed. Each has advantages and indications. 4 Third Year Internal Medicine Resident at the Hospital Universitario Fundación Santa Fe de Bogotá in We present the case of a patient with CD with localized fibrostenosis in the middle ileum which is not a Bogotá, Colombia frequent site for this type of lesion. Author for correspondence: Adriana Margarita Rey. Bogotá D.C. Colombia [email protected] Keywords ........................................ -

Adult Intussusception
1 Adult Intussusception Saulius Paskauskas and Dainius Pavalkis Lithuanian University of Health Sciences Kaunas Lithuania 1. Introduction Intussusception is defined as the invagination of one segment of the gastrointestinal tract and its mesentery (intussusceptum) into the lumen of an adjacent distal segment of the gastrointestinal tract (intussuscipiens). Sliding within the bowel is propelled by intestinal peristalsis and may lead to intestinal obstruction and ischemia. Adult intussusception is a rare condition wich can occur in any site of gastrointestinal tract from stomach to rectum. It represents only about 5% of all intussusceptions (Agha, 1986) and causes 1-5% of all cases of intestinal obstructions (Begos et al., 1997; Eisen et al., 1999). Intussusception accounts for 0.003–0.02% of all hospital admissions (Weilbaecher et al., 1971). The mean age for intussusception in adults is 50 years, and and the male-to-female ratio is 1:1.3 (Rathore et. al., 2006). The child to adult ratio is more than 20:1. The condition is found in less than 1 in 1300 abdominal operations and 1 in 100 patients operated for intestinal obstruction. Intussusception in adults occurs less frequently in the colon than in the small bowel (Zubaidi et al., 2006; Wang et al., 2007). Mortality for adult intussusceptions increases from 8.7% for the benign lesions to 52.4% for the malignant variety (Azar & Berger, 1997) 2. Etiology of adult intussusception Unlike children where most cases are idiopathic, intussusception in adults has an identifiable etiology in 80- 90% of cases. The etiology of intussusception of the stomach, small bowel and the colon is quite different (Table 1). -

Improved Postmortem Diagnosis of Taenia Saginata Cysticercosis
IMPROVED POSTMORTEM DIAGNOSIS OF TAENIA SAGINATA CYSTICERCOSIS A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfilment of the Requirements for the Degree of Masters of Science in the Department of Veterinary Microbiology University of Saskatchewan Saskatoon By WILLIAM BRADLEY SCANDRETT Keywords: Taenia saginata, bovine cysticercosis, immunohistochemistry, histology, validation © Copyright William Bradley Scandrett, July, 2007. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a postgraduate degree from the University of Saskatchewan, I agree that the libraries of this university may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make use of material in this thesis in whole or in part should be addressed to: Head of the Department of Veterinary Microbiology University of Saskatchewan Saskatoon, Saskatchewan, S7N 5B4 i ABSTRACT Bovine cysticercosis is a zoonotic disease for which cattle are the intermediate hosts of the human tapeworm Taenia saginata. -

Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital
TAJ June 2008; Volume 21 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Original Article Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital M Uddin Ahmed1, M Abdul Ahad2, M A Alim2, A R M Saifuddin Ekram3, Q Abdullah Al Masum4, Sumona Tanu5, Refaz Uddin6 Abstract A descriptive study on all cases of haematemesis and or melaena was carried out at Rajshahi Medical College Hospital to observe the demographic profile, clinical presentation, cause and outcome of upper gastrointestinal bleeding in a tertiary hospital of Bangladesh. Fifty adult patients presenting with haematemesis and or melaena admitted consecutively into medical unit were evaluated through proper history taking, thorough clinical examination, endoscopic examination with in 48 hours of first presentation and other related investigations. Patients those who were not stabilized haemodynamically with in 48 hours of resuscitation and endoscopy could not be done with in that period were excluded from this study. Results our results showed that out of 50 patients 44 were male and 6 were female and average age of the patients was 39.9 years. Most of the patients were from low socio-economic condition. Farmers, service holders and laborers were the most (57%) affected group. Haematemesis and melaena (42%), only melaena (42%) and only haematemesis (16%) were the presenting features. Endoscopy revealed that duodenal ulcer( 34%) was the most common cause of UGI bleeding followed by rupture of portal varices( 16%) , neoplasm( 10%) , gastric ulcer ( 08%) and gastric erosion( 06%). Acute upper GI bleeding is a common medical problem that is responsible for significant morbidity and mortality. -

Recognizing-Consitpation-And-Bowel
Hughes Melton, MD Post Office Box 1797 Commissioner Richmond, Virginia 23218-1797 Office of Integrated Health Health & Safety Information Dr. Dawn M. Adams DNP, ANP-BC, CHC Director, Office of Integrated Health Recognizing Constipation & Preventing Bowel Obstruction 2018 Recognizing Constipation Constipation is a disorder that occurs when bowel movements become difficult or less frequent is frequently seen in many people. Individuals with developmental disabilities often have problems with chronic constipation as a result of but not limited to: Medication side effects Neuromuscular problems related to the person's disability Signs of Constipation small infrequent bowel movements hemorrhoids due to straining with bowel movements increased abdominal girth abdominal pain Chronic constipation Chronic constipation must be addressed in all individuals. This can often be a silent problem, especially for individuals who are independent in toileting activities. Without treatment, chronic constipation can lead to bowel obstruction, bowel perforation and death. Bowel Obstruction (Intestinal Obstruction) A partial or complete block of the small or large intestine that keeps food, liquid, gas, and stool from moving through the intestines in a normal way. October 2018 Hughes Melton, MD Post Office Box 1797 Commissioner Richmond, Virginia 23218-1797 Bowel obstructions may be caused by a twist in the intestines. Intestines are called the gut. The large intestine includes the appendix, cecum, colon, and rectum and is 5 feet long. It absorbs water from stool and changes it from a liquid to a solid form. The small intestine is where most digestion occurs. It measures about 20 feet and includes the duodenum, jejunum, and ileum. o Digestion is the process of breaking down food into substances the body can use for energy, tissue growth, and repair. -

Obscure Gastrointestinal Bleeding in Cirrhosis: Work-Up and Management
Current Hepatology Reports (2019) 18:81–86 https://doi.org/10.1007/s11901-019-00452-6 MANAGEMENT OF CIRRHOTIC PATIENT (A CARDENAS AND P TANDON, SECTION EDITORS) Obscure Gastrointestinal Bleeding in Cirrhosis: Work-up and Management Sergio Zepeda-Gómez1 & Brendan Halloran1 Published online: 12 February 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Obscure gastrointestinal bleeding (OGIB) in patients with cirrhosis can be a diagnostic and therapeutic challenge. Recent advances in the approach and management of this group of patients can help to identify the source of bleeding. While the work-up of patients with cirrhosis and OGIB is the same as with patients without cirrhosis, clinicians must be aware that there are conditions exclusive for patients with portal hypertension that can potentially cause OGIB. Recent Findings New endoscopic and imaging techniques are capable to identify sources of OGIB. Balloon-assisted enteroscopy (BAE) allows direct examination of the small-bowel mucosa and deliver specific endoscopic therapy. Conditions such as ectopic varices and portal hypertensive enteropathy are better characterized with the improvement in visualization by these techniques. New algorithms in the approach and management of these patients have been proposed. Summary There are new strategies for the approach and management of patients with cirrhosis and OGIB due to new develop- ments in endoscopic techniques for direct visualization of the small bowel along with the capability of endoscopic treatment for different types of lesions. Patients with cirrhosis may present with OGIB secondary to conditions associated with portal hypertension. Keywords Obscure gastrointestinal bleeding . Cirrhosis . Portal hypertension . -

Ischaemic Colitis: Practical Challenges and Evidence-Based Recommendations For
Ischaemic Colitis: Practical Challenges and Evidence-Based Recommendations for Management Alexander Hung1*, Tom Calderbank1*, Mark A Samaan1, Andrew A Plumb2, George Webster1 Author affiliations 1Department of Gastroenterology, University College London Hospitals, London, UK 2Department of Radiology, University College London Hospitals, London, UK *Authors contributed equally Correspondence to Mark A Samaan Department of Gastroenterology University College London Hospitals 250 Euston Road London NW1 2PG [email protected] Running title Ischaemic Colitis Management Word count 3212 Key Words Ischaemic colitis, colonic ischaemia ABSTRACT Ischaemic colitis (IC) is a common condition with rising incidence and, in severe cases, a high mortality rate. Its presentation, severity and disease behaviour can vary widely and there exists significant heterogeneity in treatment strategies and resultant outcomes. In this article we explore practical challenges in the management of IC and where available, make evidence-based recommendations for its management based on a comprehensive review of available literature. An optimal approach to initial management requires early recognition of the diagnosis followed by prompt and appropriate investigation. Ideally, this should involve the input of both gastroenterology and surgery. CT with intravenous and oral contrast is the imaging modality of choice. It can support a clinical diagnosis, define the severity and distribution of ischaemia and has prognostic value. In all but fulminant cases, this should be followed (within 48 hours) by lower GI endoscopy to reach the distal-most extent of the disease, providing endoscopic (and histological) confirmation. The mainstay of medical management is conservative/supportive treatment, with bowel rest, fluid resuscitation and antibiotics. Specific laboratory, radiological and endoscopic features are recognised to correlate with more severe disease, higher rates of surgical intervention and ultimately worse outcomes. -

Patient Selection Criteria
M∙ACS MACS Patient Selection Criteria The objective is to screen, on a daily basis, the Acute Care Surgical service “touches” at your hospital to identify patients who meet criteria for further data entry. The specific patient diseases/conditions that we are interested in capturing for emergent general surgery (EGS) are: 1. Acute Appendicitis 2. Acute Gallbladder Disease a. Acute Cholecystitis b. Choledocholithiasis c. Cholangitis d. Gallstone Pancreatitis 3. Small Bowel Obstruction a. Adhesive b. Hernia 4. Emergent Exploratory Laparotomy (Refer to the ex-lap algorithm under the Diseases or Conditions section below for inclusion/exclusion criteria.) The daily census for patients admitted to the Acute Care Surgery Service or seen as a consult will have to be screened. There may be other sources to accomplish this screening such as IT and we are interested in learning about these sources from you. From this census, a list can be compiled of patients with the aforementioned diseases/conditions. The first level of data entry involves capture and entry of the patient into the MACS Qualtrics database. All patients with the identified diseases/conditions will have data entered regardless of whether or not they received an operation during admission/ED visit. The second level of data entry takes place if an existing MACS patient returns to the hospital (ED or admission) or has outcome events identified within the 30-day post-operative time frame if the patient had surgery, or within 30 days from discharge for the non-operative patients. You will see that we are capturing diagnostic, interventional, and therapeutic data that extend beyond what is typically captured for MSQC patients. -

Hematemesis and Melena Chapter
126 CHAPTER 20 Hematemesis and melena Anthony Y. B. Teoh and James Y. W. Lau Chinese University of Hong Kong, Hong Kong SAR, China ESSENTIAL FACTS ABOUT CAUSATION ESSENTIALS OF TREATMENT Algorithm for management of acute GI bleeding Diagnosis Number of patients Mortality (%) 200716 (%) Major bleeding Minor bleeding Ulcer 1826 (27) 162 (8.9) (unstable hemodynamics) Erosive disease (gastric 1731 (26) 195 (14.1) Early elective upper and duodenum) Active resuscitation endoscopy Esophagitis 1177 (17) 65 (5.5) Urgent endoscopy Varices and portal 819 (12) 87 (14) Early administration of vasoactive hypertensive drugs in suspected variceal bleeding gastropathy Active ulcer bleeding Bleeding varices Malignancy 187 (3) 31 (17) Major stigmata Mallory-Weiss 213 (3) 10 (4.7) Endoscopic therapy Endoscopic therapy Adjunctive PPI Adjunctive vasoactive syndrome drugs Other diagnosis 797 (12) 125 (16) Success Failure Success Failure Continue Continue ulcer healing Recurrent Total 6750 675 (10) vasoactive drugs medications bleeding Variceal Data adapted from The United Kingdom National Audit in Upper Repeat endoscopic eradication Gastrointestinal Bleeding 2007 [16]. therapy program Sengstaken- Success Failure Blakemore tube ESSENTIALS OF DIAGNOSIS Angiographic embolization TIPS vs vs. surgery surgery • Symptoms: Coffee ground vomiting, hematemesis, melena, hematochezia, anemic symptoms • Past medical history: Liver cirrhosis, use of non-steroidal anti- inflammatory drugs • Signs: Hypotension, tachycardia, pallor, altered mental status, and therapeutic tool in managing these patients. Stratification melena or blood per rectum, decreased urine output of the patients into low- or high-risk groups aids in formulat- • Bloods: Anemia, raised urea, high urea to creatinine ratio • Endoscopy: Ulcers, varices, Mallory-Weiss tear, erosive disease, ing a clinical management plan and early endoscopy with neoplasms, vascular ectasia, and vascular malformations aggressive post-hemostasis care should be provided in high- risk patients. -

Ischemic Colitis Caused by Polycythemia Vera: a Case Report and Literature Review
EXPERIMENTAL AND THERAPEUTIC MEDICINE 16: 3663-3667, 2018 Ischemic colitis caused by polycythemia vera: A case report and literature review SHASHA ZHANG1, RUIXUE LAI1, XUEQING GAO1, YUFEI ZHAO1, YUE ZHAO2, JIANHUA WU3 and ZHANJUN GUO1 Departments of 1Rheumatology and Immunology, and 2Gastroenterology and Hepatology, 3Animal Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050011, P.R. China Received January 16, 2018; Accepted August 10, 2018 DOI: 10.3892/etm.2018.6638 Abstract. Polycythemia vera (PV) is a chronic myeloproliferative Ischemic colitis (IC) can be described as an ischemic disease disorder originating from hematopoietic stem cells and of the colon, which results from decreased blood flow due to complicated by thrombosis and bleeding. This report describes a various causes that leads to intestinal wall ischemia, injury or case of ischemic colitis (IC) caused by PV and includes a review necrosis (3). The risk factors for IC mainly include diabetes, of the relevant literature. The patient was a 59-year-old male hypertension, dyslipidemia, peripheral vascular disease, with a history of PV who presented with abdominal pain and aspirin administration, and digoxin or constipation-inducing hematochezia. Colonoscopy and histopathological examination medications (4). The main clinical manifestations include results indicated suspected ischemic bowel disease. Following abdominal pain, diarrhea, and hematochezia (5). A reliable experimental anticoagulant therapy for 7 days, the patient no diagnosis of IC depends on the comprehensive evaluation of longer experienced abdominal pain and hematochezia had clinical symptoms, biochemical tests, radiological examina- resolved. Colonoscopy review showed no obvious anomalies tions, and endoscopic assessments (6). In certain cases, PV 1 month later. -
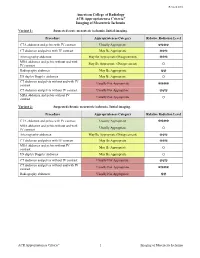
ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia Variant 1: Suspected acute mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ MRA abdomen and pelvis without and with May Be Appropriate (Disagreement) IV contrast O Radiography abdomen May Be Appropriate ☢☢ US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ MRA abdomen and pelvis without IV Usually Not Appropriate contrast O Variant 2: Suspected chronic mesenteric ischemia. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢ MRA abdomen and pelvis without and with Usually Appropriate IV contrast O Arteriography abdomen May Be Appropriate (Disagreement) ☢☢☢ CT abdomen and pelvis with IV contrast May Be Appropriate ☢☢☢ MRA abdomen and pelvis without IV May Be Appropriate contrast O US duplex Doppler abdomen May Be Appropriate O CT abdomen and pelvis without IV contrast Usually Not Appropriate ☢☢☢ CT abdomen and pelvis without and with IV Usually Not Appropriate contrast ☢☢☢☢ Radiography abdomen Usually Not Appropriate ☢☢ ACR Appropriateness Criteria® 1 Imaging of Mesenteric Ischemia IMAGING OF MESENTERIC ISCHEMIA Expert Panels on Vascular Imaging and Gastrointestinal Imaging: Michael Ginsburg, MDa; Piotr Obara, MDb; Drew L. Lambert, MDc; Michael Hanley, MDd; Michael L. Steigner, MDe; Marc A. Camacho, MD, MSf; Ankur Chandra, MDg; Kevin J. Chang, MDh; Kenneth L.