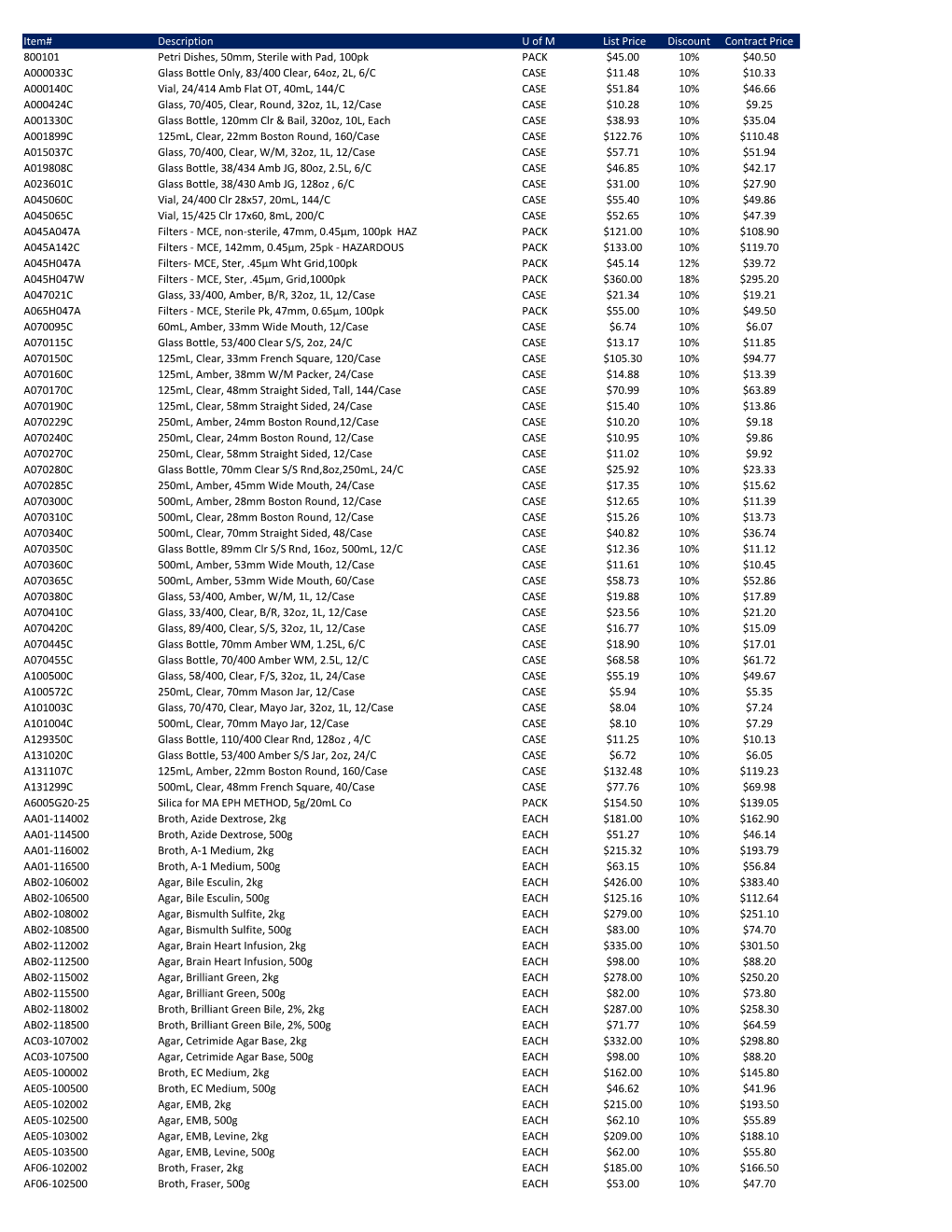
Item# Description U of M List Price Discount Contract Price
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VACUUM RECOVERY of ASPHALT EMULSION RESIDUE (An Arizona Method)
ARIZ 504 July 1980 (3 Pages) VACUUM RECOVERY OF ASPHALT EMULSION RESIDUE (An Arizona Method) Scope (d) No. 10 sieve conforming to AASHTO designation M 92. I. This method describes a low temperature vacuum procedure for recovery of the asphalt residue (e) Vacuum recovery apparatus as shown from asphalt emulsions. It is is not suitable for assembled in Fig. I. quantitative recovery of solvents from emulsions I) Vacuum source capable of producing an containing low boiling range distillates. absolute vacuum within the system of approximately 710 mm (28 in.) mercury. Apparatus 2) Thermometer - shall have a range of _5° to 1. The apparatus shall consist of the following: +200°C (23°F to 392°F). The overall length (a) Brass stirring rod. shall be 600 mm (24 in.) and the distance from the bottom of the bulb to the zero point (b) 8 oz. ointment can. shall be 300 mm (12 in.) (c) 100 ml. stainless steel beaker. 3) Stirrer hot plate. VACUUM RECOVERY APPARATUS 300 mm Allihn condenser H20 Out / Thermometer Vacuum Release Pinch Clamp 500 m. 1,000 ml. Filtration Flask Filtration Flask Teflon Stirring Bar H20 In 500 ml. Filtration Flask Portable Heat Gun FIGURE I ARIZ 504 July 1980 4) Teflon covered stirring bar. (g) Insert the stoppered themometer (positioned 5) 1000 ml. and two 500 ml. filtering flasks with in the stopper at an angle to prevent contact with tubulation. stirring bar) into the flask and set on hot plate at a medium high heat setting (#4). The bulb of the 6) 300 mm Allihn condensor. -

Temperature Distribution in a Vacuum Flask
Solved with COMSOL Multiphysics 5.0 Temperature Distribution in a Vacuum Flask Introduction The following example solves for the temperature distribution within a vacuum flask holding hot coffee. The main interest here is to illustrate how to use MATLAB® functions to define material properties and boundary condition directly within the COMSOL model. Two MATLAB functions are used to define the temperature dependent thermal conductivity of the vacuum flask shell and the insulation foam, while a third function defines the heat transfer coefficient that corresponds to a natural convective cooling for a vertical plate and surrounding air. Model Definition Assume axial symmetry for this simulation to reduce the model geometry to the 2D cross-section of the vacuum flask geometry shown in Figure 1. The vacuum flask consists of a steel shell isolated with a foam material, and a cork made of nylon. On the inside wall apply a constant temperature, assuming that the vacuum flask is filled with coffee of constant temperature. 1 | TEMPERATURE DISTRIBUTION IN A VACUUM FLASK Solved with COMSOL Multiphysics 5.0 Nylon cork Air Steel shell Constant temperature Insulation foam Figure 1: Cross section of the vacuum flask geometry Define the temperature dependent thermal conductivity of steel according to the following polynomial expression: –4 2 –8 3 ksteel = 71.12– 0.115T + 1.16e T – 4.25e T As shown in Figure 2, in the temperature interval of interest for the model, the thermal conductivity decreases with increasing temperature. 2 | TEMPERATURE DISTRIBUTION IN A VACUUM FLASK Solved with COMSOL Multiphysics 5.0 Figure 2: Thermal conductivity of the steel wall versus temperature. -

Liquefaction, Storage and Transfer of Cryofluids
Storage and transfer of cryofluids Christian Gianese www.neel.cnrs.fr Guilty 1: Arsène d’Arsonval (1851 - 1940) He invented a glass container with double wall, the vacuum being done in the space between the outer and inner walls: the vase d'Arsonval. About 1902, he collaborated with Georges Claude on the liquefaction of gases and inspires industries Air Liquid. www.neel.cnrs.fr Guilty 2: James Dewar (1842 - 1923) He discovered a process to produce liquid oxygen in 1891 and liquid hydrogen in 1898, in industrial quantities. He developed an insulating bottle, the Dewar flask, still named after him, to study low temperature gas phenomena. In fact, he improves the d’Arsonval vessel by depositing a layer of silver on the inside wall, to minimize the heat input by radiation. He also used this bottle to transport liquid gases such as hydrogen. In 1905, he observed that cold charcoal could produce a vacuum. www.neel.cnrs.fr Thermos The first vacuum flasks for commercial use were made in 1904 when a german company, Thermos GmbH, was formed. Thermos, their tradename for their flasks, remains a registered trademark in some countries but was declared a genericized in the US in 1963 as it is colloquially synonymous with vacuum flasks in general; in fact it is far more common to speak of a domestic thermos than a vacuum flask. www.neel.cnrs.fr STORAGE www.neel.cnrs.fr The most important factors in storage systems Logistics Volume of storage, dimensions, transport, etc Reliability, safety Economics ! Rate of evaporation of cryogenic liquids (helium: -

Organic Chemistry Ii
University of Maribor Faculty of Chemistry and Chemical Engineering Laboratory for Organic and Polymer Chemistry and Technology Laboratory Course ORGANIC CHEMISTRY II Muzafera Paljevac and Peter Krajnc Proofreader: Dr. Victor Kennedy 1. THE LIST OF LABORATORY EXPERIMENTS IN ORGANIC CHEMISTRY II LAB COURSE 1. Determination of melting point 2. Continuous (fractional) distillation 3. Distillation with water steam 4. Recrystallization, Sublimation 5. Paper and thin layer chromatography _____________________________________________________________________________________ 6. Synthesis of acetylsalicylic acid 7. Synthesis of tert-butyl chloride 8. Synthesis of methyl orange 9. Synthesis of aniline 10. Synthesis of ethyl acetate 11. Synthesis of ethyl iodide 1 2. LABORATORY RULES AND REGULATIONS - You must wear a lab coat at all times when working in the laboratory. You are expected to provide your own lab coat, and you will not be allowed to work in the lab without one. - Safety glasses and gloves will be supplied when required and must be worn where notices, experimental instructions or supervisors say so. - Long hair must be tied back when using open flames. - Eating and drinking are strictly prohibited in the laboratory. - Coats, backpacks, etc., should not be left on the lab benches and stools. There are coat racks just outside the lab. Be aware that lab chemicals can destroy personal possessions. - Always wash your hands before leaving the lab. - Notify the instructor immediately in case of an accident. - Before leaving the laboratory, ensure that gas lines and water faucets are shut off. - Consider all chemicals to be hazardous, and minimize your exposure to them. Never taste chemicals; do not inhale the vapors of volatile chemicals or the dust of finely divided solids, and prevent contact between chemicals and your skin, eyes and clothing. -

Simple Calorimeter for Heats of Fusion. Data on The
U.S. Department of Commerce, Bureau of Standards RESEARCH PAPER RP607 Part of Bureau of Standards Journal of Research, Vol. 11, October 1933 A SIMPLE CALORIMETER FOR HEATS OF FUSION. DATA ON THE FUSION OF PSEUDOCUMENE, MESITYLENE (« AND 0), HEMIMELLITENE, o- AND m-XYLENE, AND ON TWO TRANSITIONS OF HEMIMELLITENE By Frederick D. Rossini abstract A vacuum flask with a thermoelement serves as a simple calorimeter for measuring heats of fusion quickly and economically, with an accuracy of a few percent. The following heats of fusion (with estimated uncertainties), in k-cal. per mole, were obtained: pseudocumene, — 44.1° C, 2.75±0.06; hemimelli- tene,-25.5° C, 2.00±0.05; mesitylene (a), — 44.8° C, 2.28±0.06; mesitylene (0), -51.7° C., 1.91±0.05; o-xylene,-25.3° C, 3.33±0.07; m-xylene,-47.9° C, 2.76 ±0.05. Hemimellitene was found to have two transitions below the freez- ing point, with the following heats of transition, in A>cal. per mole: hemimelli- tene (7-»0),-58±2° C.,0.28±0.04; hemimellitene (P^a),- 46 ± 1° C.,0.36±0.04. CONTENTS Page I. Introduction 553 II. Apparatus and method 553 III. Materials 554 IV. Standardization experiments 555 V. Experimental data 557 VI. Conclusion 559 I. INTRODUCTION The simple calorimeter described here was assembled in order to provide a means for measuring as quickly and economically as prac- ticable, and with an accuracy of a few percent, the heats of fusion of certain hydrocarbons for which there are no data. -

APR PET S-12 Dissolution Screening
PET Dissolution Test Document Code – PET-S-12 Publication or Revision Date: November 16, 2018 Introduction – Scope, Significance and Use This protocol is derived from a copyrighted method owned by M&G Polymers USA LLC and international copyright treaties protect the method. In consideration for the use of this method, users agree to hold APR and M&G Polymers USA LLC and its affiliated companies harmless and not be liable for any incidental, consequential or other indirect damages resulting from the use of this method. Disclaimer: This document has been prepared by the Association of Plastic Recyclers as a service to the plastic industry to promote the most efficient use of the nation’s plastic recycling infrastructure and to enhance the quality and quantity of recycled postconsumer plastic. The information in this document is offered without warranty of any kind, either expressed or implied, including WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, which are expressly disclaimed. APR and its members accept no responsibility for any harm or damages arising from the use of or reliance upon this information by any party. Participation in the Recognition Program is purely voluntary and does not guarantee compliance with any U.S. law or regulation or that a package or plastic article incorporating the innovation is recyclable or will be recycled. Equipment/Supplies List • Heat resistant gloves • Apron; full length that covers torso, arms and legs • Faceshield (hood sash if available) • Metal beaker with handle, at least 700 ml capacity • Fiberglass matting for setting hot beaker on while heating and cooling • Thermometer with metal armor casing • Safety glasses • Vented Hood • Hot Plate • 1000 ml filtering flask • Buchner funnel • Filter Paper (Whatman #1, 11 micron, VWR Catalog #28450-128) ©2018 Association of Plastic Recyclers. -

High School Chemistry
RECOMMENDED MINIMUM CORE INVENTORY TO SUPPORT STANDARDS-BASED INSTRUCTION HIGH SCHOOL GRADES SCIENCES High School Chemistry Quantity per Quantity per lab classroom/ Description group adjacent work area SAFETY EQUIPMENT 2 Acid storage cabinet (one reserved exclusively for nitric acid) 1 Chemical spill kit 1 Chemical storage reference book 5 Chemical waste containers (Categories: corrosives, flammables, oxidizers, air/water reactive, toxic) 1 Emergency shower 1 Eye wash station 1 Fire blanket 1 Fire extinguisher 1 First aid kit 1 Flammables cabinet 1 Fume hood 1/student Goggles 1 Goggles sanitizer (holds 36 pairs of goggles) 1/student Lab aprons COMPUTER ASSISTED LEARNING 1 Television or digital projector 1 VGA Adapters for various digital devices EQUIPMENT/SUPPLIES 1 box Aluminum foil 100 Assorted rubber stoppers 1 Balance, analytical (0.001g precision) 5 Balance, electronic or manual (0.01g precision) 1 pkg of 50 Balloons, latex 4 Beakers, 50 mL 4 Beakers, 100 mL 2 Beakers, 250 mL Developed by California Science Teachers Association to support the implementation of the California Next Generation Science Standards. Approved by the CSTA Board of Directors November 17, 2015. Quantity per Quantity per lab classroom/ Description group adjacent work area 2 Beakers, 400 or 600 mL 1 Beakers, 1000 mL 1 Beaker tongs 1 Bell jar 4 Bottle, carboy round, LDPE 10 L 4 Bottle, carboy round, LDPE 4 L 10 Bottle, narrow mouth, 1000 mL 20 Bottle, narrow mouth, 125 mL 20 Bottle, narrow mouth, 250 mL 20 Bottle, narrow mouth, 500 mL 10 Bottle, wide mouth, 125 -

Mycoplasma Screening of Cultured Cells by Fluorescence Microscopy
Mycoplasma Screening of Cultured Cells by Fluorescence Microscopy Prepare cell samples Make sure cells have been passages at least 2 times after a thaw so mycoplasma have recovery time from their time in cold storage!! 1. With sterile tweezers or Pasteur pipet attached to vacuum flask place one 22x22mm or 25mm round sterile glass coverslip into well of sterile 6well plate (# wells = # cell lines tested) or 35mm dish. 2. Add 3 ml of (cell line specific) complete medium without antibiotics (important because they may inhibit mycoplasma and produce a false negative) slowly to the well (touch first drop of medium to the edge of the coverslip until capillary action carries the liquid under the coverslip and completely covers the underside, which holds the coverslip to the dish and disallows cell growth on the underside). 3. Add 2-4 drops of cell suspension (approximately 10^5cells) to the medium in the well. Rock gently. (Note: For testing non-adherent cells, a negative indicator line must be used. The line to be tested must be coplated with the indicator line. Mycoplasma will transfer to the indicator line within 2 days and will be visualized.) 4. Incubate 2-3 days at appropriate temperature for cell line until they are approximately 70% confluent (mycoplasma live on the outer membrane, and thus are easier to visualize when cells are subconfluent). To Fix 1. Add 3 ml methanol gently to the side of the dish (do not remove growth medium). 2. Leave for 3 min at room temperature. 3. Aspirate methanol/medium solution. 4. Add 3 ml methanol for 15 min at room temperature. -

Edna PROTOCOL SAMPLE COLLECTION
eDNA PROTOCOL SAMPLE COLLECTION Caren Goldberg and Katherine Strickler, Washington State University September 2014 Adapted from Protocol Version 04/12/2012 (D.S. Pilliod, R.S. Arkle, and M.B. Laramie) USGS Snake River Field Station MATERIALS 1. Cellulose nitrate disposable filter funnels or other field-tested, disposable filter funnels 2. Vacuum flask (1L) 3. Silicone tubing 4. Vacuum hand pump (from auto parts store) 5. Rubber stopper with hole for funnel stem 6. Latex or nitrile gloves (non-powdered) 7. Forceps (filter forceps if possible) 8. High quality, o-ring screw cap 2mL tubes (e.g., Sarstedt brand) with 1mL 100% molecular-grade ethanol (not denatured) 9. Ethanol-proof laboratory pen (do not use a regular Sharpie marker) 10. 50 mL tubes of 30 mL 1) 50% household bleach solution 2) distilled water in a holder (a foam drink holder [koozie] works well) 11. Polypropylene grab bottles and cooler (for off-site filtering) or Whirl-Pak® bags (for on- site filtering) 12. Water, bleach, scrub brush, and tubs (for decontaminating between sites) 2 3 5 1 4 2 Figure 1. Filter funnel (1), vacuum flask (2), silicone tubing (3), vacuum pump (4), and rubber stopper (5). 9 7 10 8 6 1 Figure 2. Latex or nitrile gloves (6), forceps (7), 2 mL tubes with 1 mL ethanol (8), ethanol-proof lab marker (9), and 2 50 mL tubes with 50% household bleach solution distilled water (10). 3 11a 11b Figure 3. Polypropylene grab bottles (11a) and Whirl-Pak® bag (11b). 12a 12d 12b 12c Figure 4. Water (12a), bleach (12b), scrub brushes (12c), and tubs (12d) for decontaminating boots and equipment between sites. -

Forms of Energy
Plot 48 Muwayire Rd (behind IHK Hospital) P.O.BOX 5337, KAMPALA - UGANDA Tel: 256783111908 Email: [email protected] Website: www.stagnes.co.ug FORMS OF ENERGY Energy is the body’s ability to do work Matter Matter is anything that has mass and volume OR anything that has weight and occupies space The meaning of each of the following a. Mass: is a quantity of matter in an object. b. Volume: Is the space occupied by an object. c. Molecules: It is the smallest particles of matter. d. Weight: is the gravitational force exerted on an object by the earth. States of matter There are three different states of matter - Solids - Liquids - Gases Solids Examples of solids. - Wood - Rubber - Glass - Plastic Characteristics of solids. - Molecules are closely packed. - Particles are held together very tightly. 1 - Molecules do not move from position but vibrate. - Solids have shape, size and volume apart from irregular objects. Diagram to show the arrangement of molecules. Liquids Examples of liquids. - Water - Soda - Oils - Juice Characteristics of liquids. - Molecules are spaced. - Molecules loosely held together. - Liquids have a proper volume (capacity) - Liquids have no definite shape (take up the shape of the container in which they are poured) Diagram to show the arrangement of molecules. Gases Examples of gases. - Nitrogen - Carbon dioxide - Oxygen 2 - Rare gases Characteristics of gases. - Molecules are far apart - Gases have no definite shape - Gases have a particular volume. - Molecules move freely. Diagram to show the arrangement of molecules. ACTIVITY 1. What is energy? 2. What is matter? 3. Name the three states of matter. -

Basic Cell Culture Techniques
BASIC CELL CULTURE TECHNIQUES STERILE TECHNIQUES Aseptic or sterile technique is the execution of tissue culture procedures without introducing contaminating microorganisms from the environment. In doing tissue culture work, 70% of the problems are due to a lack of good sterile technique. Microorganisms causing the contamination problems exist everywhere, on the surface of all objects and in the air. A conscious effort must be made to keep them out of a sterile environment. Because many and sometimes awkward manipulations are required for various techniques, tissue culture media used are often supplemented with antibiotics. Antibiotics do not eliminate problems of gross contamination which result from poor sterile technique or antibiotic- resistant mutants. Autoclaving renders pipettes, glassware, and solutions sterile. Nutrient medium cannot be autoclaved. The compounds in nutrient medium are destroyed by the heat of autoclaving. Medium must therefore be sterilized by passing it through a sterile filter small enough in pore size to hold back bacteria and mycoplasmas (Millipore Sterivex - GS 0.22u disposable filter units). Here are some rules of thumb to follow to keep your medium, cultures, and glassware from becoming contaminated: 1. Wipe your work area and hands with 70% ethanol before starting. Spray any container you plan to put into the sterile workspace with 70% ethanol. 2. Never uncover a sterile flask, bottle, petri dish, etc., until the instant you are ready to use it. Return the cover as soon as you are finished. Never leave it open to the environment. 3. Sterile pipettes should never be taken from the wrapper until they are to be used. -

Chemistry 2A Lab Manual Standard Operating Procedures Winter Quarter 2018
Chemistry 2A Lab Manual Standard Operating Procedures Winter Quarter 2018 Department of Chemistry University of California - Davis Davis, CA 95616 Student Name Locker # Laboratory Information Teaching Assistant’s Name Laboratory Section Number Laboratory Room Number Dispensary Room Number 1060 Sciences Lab Building Location of Safety Equipment Nearest to Your Laboratory Safety Shower Eye Wash Fountain Fire Extinguisher Fire Alarm Safety Chemicals Revision Date 12/1/2017 Preface Chemistry is an experimental science. Thus, it is important that students of chemistry do experiments in the laboratory to more fully understand that the theories they study in lecture and in their textbook are developed from the critical evaluation of experimental data. The laboratory can also aid the student in the study of the science by clearly illustrating the principles and concepts involved. Finally, laboratory experimentation allows students the opportunity to develop techniques and other manipulative skills that students of science must master. The faculty of the Chemistry Department at UC Davis clearly understands the importance of laboratory work in the study of chemistry. The Department is committed to this component of your education and hopes that you will take full advantage of this opportunity to explore the science of chemistry. A unique aspect of this laboratory program is that a concerted effort has been made to use environmentally less toxic or non-toxic materials in these experiments. This was not only done to protect students but also to lessen the impact of this program upon the environment. This commitment to the environment has presented an enormous challenge, as many traditional experiments could not be used due to the negative impact of the chemicals involved.