Noninvasive Collection of Saliva in Panthera
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Welcome to Syracuse
WELCOME TO SYRACUSE As you begin your new journey in Syracuse, we have included some information that you may find helpful as you adjust to your new home. Inside you will find information about our city to jumpstart your Syracuse experience. CLIMATE & WEATHER SNAPSHOT OF SYRACUSE! Experience four distinct The city of Syracuse is located in Onondaga County seasons in the geographic center of New York State. The Average Temperatures: Onondaga, Syracuse Metropolitan Area is made up of Cayuga, Madison, Onondaga, and Oswego counties. Area Code: 315 Population in 2021: City of Syracuse: 141,491 Onondaga County: 458,286 Median Age: Syracuse: 30.6 September: Onondaga County: 39 64 degrees New York State: 38.2 United States: 38.2 The Heart of New York From Syracuse, it’s easy to venture Montreal Ottawa out to explore the state, as well CANADA Burlington January: as major eastern cities. VERMONT Toronto NEW YORK 24 degrees NEW Nearby Distance Rochester HAMPSHIRE Buffalo SYRACUSE Boston Major Cities by Miles Albany Binghamton MASSACHUSETTS Hartford Albany, NY 140 miles RHODE CONNECTICUT ISLAND Baltimore, MD 300 miles Cleveland PENNSYLVANIA OHIO Newark New York City Binghamton, NY 75 miles Pittsburgh Philadelphia Boston, MA 300 miles NEW JERSEY Buffalo, NY 150 miles WEST Baltimore VIRGINIA Chicago, IL 665 miles Washington, DC DELAWARE Cleveland, OH 330 miles VIRGINIA MARYLAND Montreal, QC 250 miles New York, NY 260 miles Niagara Falls, NY 165 miles Philadelphia, PA 255 miles #54 Best National Pittsburgh, PA 345 miles Universities Rochester, NY 85 miles ~ US News & World Report Toronto, ON 250 miles July: Washington, DC 350 miles 72 degrees TRANSPORTATION There are many options to navigate the city, even if you don’t have a car. -

Selection of Trees Marked by Rubbing by Andean Bears in the Peruvian Dry Forest
Master’s Thesis 2016 60 credits Department of Ecology and Natural Resource Management Selection of Trees Marked by Rubbing by Andean Bears in the Peruvian Dry Forest Jack Kleiner Tropical Ecology and Natural Resource Management Preface This project was funded by the International Association for Bear Research and Management under a conservation and research grant. I am greatly thankful to Fred Dean and the team at the IBA for their support and patients throughout my project. I would like to give a special thanks to my supervisor Jon Swenson for keeping an eye on me throughout my project, helping me stick to my deadlines, and providing friendly support along the way. Secondly I would like to thank my co-supervisor Sam Steyaert, for providing much needed guidance, and a lot of time and effort to help me understand the world of bears and statistical analysis. Thank you Russ Van Horn for helping to design the project and actually getting me into the field. I am also thankful for the endless support via email throughout my project. I am very thankful for the flexibility of Robyn Appleton for allowing me to collect data from Cerro de Venado at such short notice. My deepest appreciation and thanks to the Velajos family and SBC for their hospitality and warmth. I would like to give a huge thank you to Javier Velajos, Jose Velajos, and Russ Van Horn for help collecting data. Thank you Richard Bischof, Anne Hertel, and Raju Rimal for assistance with statistics. i SELECTION OF TREES MARKED BY RUBBING BY ANDEAN BEARS IN THE PERUVIAN DRY FOREST Jack D. -

2020 Annual Report
2020 ANNUAL REPORT Providing a level of excellence that makes the Rosamond Gifford Zoo a national leader in animal care, conservation and visitor experience. 1 A JOINT MESSAGE FROM THE EXECUTIVE DIRECTOR AND THE CHAIR OF THE BOARD TABLE OF CONTENTS Facebook followers increased from 61.3K at the start The year 2020 was undoubtedly the most challenging in our history. However, we can celebrate of 2020 to 65.8K on many successes which proved that perseverance, teamwork and, most importantly, a supportive December 31, 2020, adding community can see us through anything. Navigating a Pandemic 4 followers. Over the past year, our amazing Friends of the Zoo community truly went above and beyond for 4,500 Maintaining Partnerships your zoo. You let us know how much you missed visiting while we were closed, you came back 9 Surpassed as soon as you could, and you contributed to several campaigns to help the zoo recover from the 10 Engaging our Community pandemic. 25,000 Capital Improvements followers on Instagram, When we substituted a fundraising campaign - $50K for 50 Years – for a Friends of the Zoo 50th 12 a huge milestone. anniversary celebration, you pitched in to help us raise more than $20,000 over our $50,000 goal. When we offered a two-month extension on memberships to cover the COVID closure, most 13 2020 Accomplishments of you donated it back to the zoo. When we asked our volunteers to help the zoo acquire more flamingos to expand our flock, you donated to the Fund for Flamingo Flamboyance. Or, you gave 14 Development and Fundraising to our Annual Appeal on behalf of a baby patas monkey named Iniko -- “born during troubled Nearly 9,140 times.” 15 New Leadership children and adults actively participated in conservation education learning programs When, at the end of an already difficult year, we lost our two youngest elephants to another 16 Future Focus deadly virus, you mourned with us, sent messages of encouragement and donated to the Ajay and Batu Memorial Fund to help the new Animal Health Center test for and treat EEHV. -

City of Syracuse Consolidated Plan Year 30 2004-2005
Mayor Matthew J. Driscoll City of Syracuse Consolidated Plan Year 30 2004-2005 Prepared by the Department of Community Development 201 E. Washington Street, Room 612 Syracuse, New York 13202 April 2004 Consolidated Plan 2004-2005 Public Comments Consolidated Plan 2004-2005 Public Hearing March 29, 2004 Consolidated Plan 2004-2005 Newspaper Articles Draft Consolidated Plan 2004-2005 Announcement of Availability Draft Consolidated Plan 2004-2005 Public Meeting March 10, 2004 Community Needs Meeting October 23, 2003 Request for Proposals September 2003 Table of Contents 2004-2005 (Year 30) Consolidated Plan Page No. Executive Summary 1 Section 1 Introduction 10 Purpose 11 Key Participants 13 Citizen Participation 18 Community Profile 35 Section 2 Housing Market Analysis 41 Housing Market Study 42 Syracuse Housing Authority 45 Assisted Housing 55 Continuum of Care Strategy 61 Barriers to Affordable Housing 76 Fair Housing Initiatives 79 Section 3 Housing and Homeless Needs Assessment 90 Housing Needs 91 Housing Needs Assessment 92 Homeless Population Needs 99 Special Needs Population 106 Section 4 Economic Development 112 Section 5 Historic Preservation 124 Collaborative Preservation Efforts 125 Historic Preservation: 290 E. Onondaga Street 126 Section 6 Strategic Plan 140 Housing Strategic Plan 141 Homeless Strategic Plan 146 Community Development Strategic Plan 147 Public Facilities 148 Planning & Administration 149 Infrastructure 150 Public Services 151 Economic Development 152 Barriers to Affordable Housing 156 Lead-Based Paint Hazards -

References L
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Northern Prairie Wildlife Research Center Wildlife Damage Management, Internet Center for 2003 References L. David Mech USGS Northern Prairie Wildlife Research Center, [email protected] Luigi Boitani University of Rome, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsnpwrc Part of the Animal Sciences Commons, Behavior and Ethology Commons, Biodiversity Commons, Environmental Policy Commons, Recreation, Parks and Tourism Administration Commons, and the Terrestrial and Aquatic Ecology Commons Mech, L. David and Boitani, Luigi, "References" (2003). USGS Northern Prairie Wildlife Research Center. 320. https://digitalcommons.unl.edu/usgsnpwrc/320 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Northern Prairie Wildlife Research Center by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. References Abrams, P. A. 2000. The evolution of predator-prey interactions. Adams, L. G., B. W. Dale, and L. D. Mech. 1995. Wolf predation on Annu. Rev. Ecol. Syst. 31:79-105. caribou calves in Denali National Park, Alaska. Pp. 245-60 Abuladze, K. I. 1964. Osnovy Tsestodologii. Vol. IV. Teniaty in L. N. Carbyn, S. H. Fritts, and D. R. Seip, eds., Ecology lentochnye gel' minty zhivotnykh i cheloveka i vyzyvaevaniia. and conservation of wolves in a changing world. Canadian Nauka, Moscow. 530 pp. Circumpolar Institute, Edmonton, Alberta. Achuff, P. L., and R. Petocz. 1988. Preliminary resource inventory Adams, L. G., F. G. Singer, and B. W. -

Olfactory Environmental Enrichment of Felids and the Potential Uses of Conspecific Odours
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Institute of Veterinary, Animal and Biomedical Sciences Massey University PALMERSTON NORTH NEW ZEALAND Olfactory environmental enrichment of felids and the potential uses of conspecific odours A thesis presented m partial fulfilment of the requirem ents for th e degree of of Masters of Sc ience In Zo ology at Mass ey University Heidi Roesch 2003 I Thesis Abstract The potential of olfactory stimulation as a tool for the enviro nmental enrichment of captive felids was investigated at Orana Wildlife Park in Christchurch. Six cheetah (Acyninoxjubatus), two serval (Felis serval) and one tiger (Panthera tigris) were given various scents: male domestic cat urine; a synthetic analogue of domestic cat facial pheromone; mouse odour; peppermint and catnip, in order to determine whether scent as an enviro nmental enrichment can effectively modify felid behaviour. All of the scents elicited a response that was significantly different to the control presentation. The synthetic fe line facial pheromone elicited the greatest response, particularly from the fe males in the study. However, despite these results, the interest shown in the scents was limited, and due to the small sample size and other constrictions that arise fromworking with a zoo, the effectiveness of scent as a tool for environmental enrichment remains inconclusive and furtherresea rch is needed. The further possibilities of scent as an environmental technique were investigated at Massey University's Feline Nutrition Unit. -
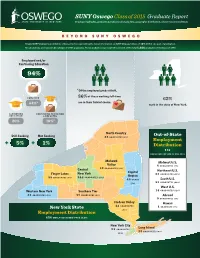
SUNY Oswego Class of 2015 Graduate Report Employer Highlights, Graduate & Professional Study Data, Geographic Distribution, Alumni Recommendations
SUNY Oswego Class of 2015 Graduate Report Employer highlights, graduate & professional study data, geographic distribution, alumni recommendations BEYOND SUNY OSWEGO Beyond SUNY Oswego is an initiative of Career Services providing the latest information on SUNY Oswego’s Class of 2015 within one year of graduation. All calculations are based on knowledge of 1,057 graduates. These graduates represent 65% percent of the total 1,626 graduates of the Class of 2015. Employed and/or Continuing Education 94% *Of the employed grads at left, of those working full-time EMPLOYED 86% 62% are in their field of choice. 64%* work in the state of New York. CONTINUING CONTINUING EDUCATION EDUCATION & EMPLOYED 20% 10%* North Country Still Seeking Not Seeking 28 GRADUATES (4%) Out-of-State Employment + 5% + 1% Distribution 116 EMPLOYED OUTSIDE OF NYS (11%) Mohawk Midwest U.S. Valley 8 GRADUATES (7%) Central 28 GRADUATES (4%) Capital Northeast U.S. Finger Lakes New York GRADUATES (28%) Region 33 58 GRADUATES (9%) 243 G R ADUATE S (37%) 45 GRADS South U.S. GRADUATES (44%) (7%) 51 West U.S. Western New York Southern Tier 14 GRADUATES (12%) 23 GRADUATES (3%) 37 GRADUATES (6%) Abroad 9 GRADUATES (8%) Hudson Valley Hawaii 43 GRADUATES 1 GRADUATE (1%) New York State (7%) Employment Distribution 658 EMPLOYED IN NEW YORK STATE New York City Long Island 98 GRADUATES 55 GRADUATES (8%) (15%) 678 Graduates • Information on 424 (62.5%) Top Advice College of Liberal Arts 232 Employed (54.7%) • 115 Graduate School (27.1%) from the and Sciences 38 Employed & Graduate School -

Sleep Inn & Suites Airport East Syracuse, NY 13057
Sleep Inn & Suites Airport East Syracuse, NY 13057 A family developed, owned and operated hotel since 2016. GREGG SARGENT | DIRECTOR 631-553-2917 [email protected] This listing is property of Baron Realty Group and is only intended for the current recipient. If you received this listing through an unauthorized representative, please disregard. Baron Realty Group has been chosen to exclusively market for sale the Sleep Inn & Suites located at 6344 E Molloy Road in East Syracuse conveniently located off NYS Thruway 90, US 81 and Route 481. This 3 story elevator building with 54 spacious rooms and suites was newly developed two years ago. This full service hotel property is one mile from the Syracuse Hancock International Airport and only minutes from downtown Syracuse, the Carrier Dome and Syracuse University. This is a true turn-key opportunity to acquire a new hospitality asset and established business in a stable New York market. The hotel contains two kitchens, a restaurant with a full bar and boasts 4 banquet rooms for business meetings, weddings and corporate events of all sizes. The large courtyard located just outside the bar is equipped with natural gas fire pits and heat lamps providing a great outdoor space to accommodate guests year around. 6344 E Molloy Road East Syracuse, New York 13057 Purchase Price $7,900,000 Property Improvement Plan $0 Total Investment $7,900,000 Total Number of Rooms 54 Occupancy 67.3% Year Built 2016 Number of Stories 3 Type of Ownership Fee Simple Financial data provided upon execution of CA from Baron Realty Group LLC. -

City of Syracuse Consolidated Plan 5 Annual Action Plan 2009-2010
Mayor Matthew J. Driscoll City of Syracuse Consolidated Plan 5th Annual Action Plan 2009-2010 Fernando Ortiz, Jr., Commissioner Department of Community Development 201 E. Washington Street, Room 612 Syracuse, New York 13202 May 2009 SF 424 The SF 424 is part of the CPMP Annual Action Plan. SF 424 form fields are included in this document. Grantee information is linked from the 1CPMP.xls document of the CPMP tool. SF 424 Complete the fillable fields (blue cells) in the table below. The other items are pre-filled with values from the Grantee Information Worksheet. 03/19/2009 Applicant Identifier Type of Submission Date Received by state State Identifier Application Pre-application Date Received by HUD Federal Identifier Construction Construction Non Construction Non Construction Applicant Information City of Syracuse NY366376 SYRACUSE Department of Community Development Organizational DUNS 07-160-7675 201 E Washington St, Room 612 Organizational Unit Syracuse New York Department 13202 Country U.S.A. Division Employer Identification Number (EIN): Onondaga County 15-60000416 Program Year Start Date (MM/DD) Applicant Type: Specify Other Type if necessary: Local Government: City Specify Other Type U.S. Department of Program Funding Housing and Urban Development Catalogue of Federal Domestic Assistance Numbers; Descriptive Title of Applicant Project(s); Areas Affected by Project(s) (cities, Counties, localities etc.); Estimated Funding Community Development Block Grant 14.218 Entitlement Grant CDBG Project Titles Description of Areas Affected -

2019 ANNUAL REPORT a National Leader in Animal Care, Conservation and Visitor Experience
Providing a level of excellence that makes the Rosamond Gifford Zoo 2019 ANNUAL REPORT a national leader in animal care, conservation and visitor experience. 1 A JOINT MESSAGE FROM THE INTERIM EXECUTIVE DIRECTOR AND THE CHAIR OF THE BOARD There is much to celebrate in the Friends 2019 annual report. Last year saw the birth of a baby Asian elephant and two critically endangered Amur leopard cubs. The zoo unveiled several new exhibits and welcomed several new rare and endangered species. Friends of the Zoo and our county partners upgraded and expanded the Helga Beck Asian Elephant Preserve, and construction began on a key Friends project, the Zalie & Bob Linn Amur Leopard Woodland. Some 332,000 people visited the zoo, enhanced by the Friends’ addition of a summer-long Big Bugs! attraction and a new 18-horse carousel. Our education programs involved more than 10,000 participants, including more than 7,500 school-age children. Our volunteers provided educational enrichment to guests, beautified the zoo with lush gardens and joined work projects to improve the facility. Our catering staff executed more than 120 events, from weddings to the Snow Leopard Soirée to Brew at the Zoo. All told, Friends provided more than $175,000 in direct support to the zoo and allocated more than $500,000 for capital improvements to help the zoo meet the “gold standard” for animal care and welfare, conservation education and saving species. We are extremely proud of these accomplishments, and we hope you will enjoy this look back at our achievements and all the people, businesses and community partners that helped make them possible. -

Thesis a Noninvasive Method Using Auditory Predator
THESIS A NONINVASIVE METHOD USING AUDITORY PREDATOR CALLS AND HAIR SNARES TO DETECT AND GENETICALLY SAMPLE COUGARS (Puma concolor) Submitted by Kirstie L. Yeager Graduate Degree Program in Ecology In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Spring 2016 Master’s Committee: Advisor: William L. Kendall Mathew W. Alldredge Kevin R. Crooks W. Chris Funk Copyright by Kirstie L. Yeager 2016 All Rights Reserved ABSTRACT A NONINVASIVE METHOD USING AUDITORY PREDATOR CALLS AND HAIR SNARES TO DETECT AND GENETICALLY SAMPLE COUGARS (Puma concolor) A noninvasive method that will sample all individuals in a population over multiple occasions is a useful tool in assessing population demographics with little disturbance to the target animals; however, finding such a method for large carnivores, such as cougars, is challenging due to their elusive nature and large home-range sizes. Current methods to sample cougars usually involve a physical capture component, but obtaining reliable estimates can be difficult and cost prohibitive when using capture as the sole sampling method. Because cougars leave sign, and exhibit behaviors like territoriality and curiosity, a noninvasive-genetic-sampling (NGS) method may be a plausible alternative. Hair contains DNA, which can be genetically analyzed to yield the individual identification necessary for population assessments and can be obtained without handling the animal. I tested NGS techniques using attractants, specifically scent lures and auditory calls, and hair snares to sample cougars at lure sites on the Front Range, Colorado during February – April, 2012 and November, 2012 – April, 2013. First, I established 16 – 20 sites over four ≈ 30-day sampling periods. -
Connect-Card-Directory.Pdf
Connect Card directory One great card can connect you to: • WCNY Event ticket discounts. • Restaurants and attractions 2-for-1 dining, admissions discounts, or special benefits. CONNECT CARD BENEFITS BY LEVEL • Bronze Level Discounts on WCNY events including live screenings, concerts, dining, and attractions. • Silver, Gold, and Platinum Levels Bronze benefits plus special invitations to WCNY events. CONNECT CARD FAQs How to find the most current list of restaurants & attractions? New partners are added regularly. For complete descriptions, sample menus, photos and other information, Visit wcny.org/connectcard or use the Connect Card App on your smart phone. How to use Connect Card? Simply present your Connect Card to your server when paying your bill or ticket agent when purchasing admission. The associated code number on the back of your card will be checked off, confirming that you have received your benefit. Search “WCNY Connect Card” App on your smartphone and a create a profile. Redeem using the App: 1. Log into account 2. Find provider page and check the benefits, restrictions and other details 3. Hit the Redeem button 4. Show the redeem code to server or ticket agent 5. You will receive the redemption information through email *Please check the restaurant/attraction profiles to understand any restrictions that may be applied when using your Connect Card. Unless otherwise stated, benefits are single use only. When redeeming 2-for-1 benefits, the lower cost item will be removed from your bill. Additional restrictions may apply, so please read the benefits offer before purchasing. 2 Connect Card 5 Zones Includes Southern Canada 2 1 6 4 Includes 7 Long Island 3 8 Includes All Other Areas For the most updated list of Connect Card partners, visit wcny.org/connectcard or download the app on your Apple or Android device.