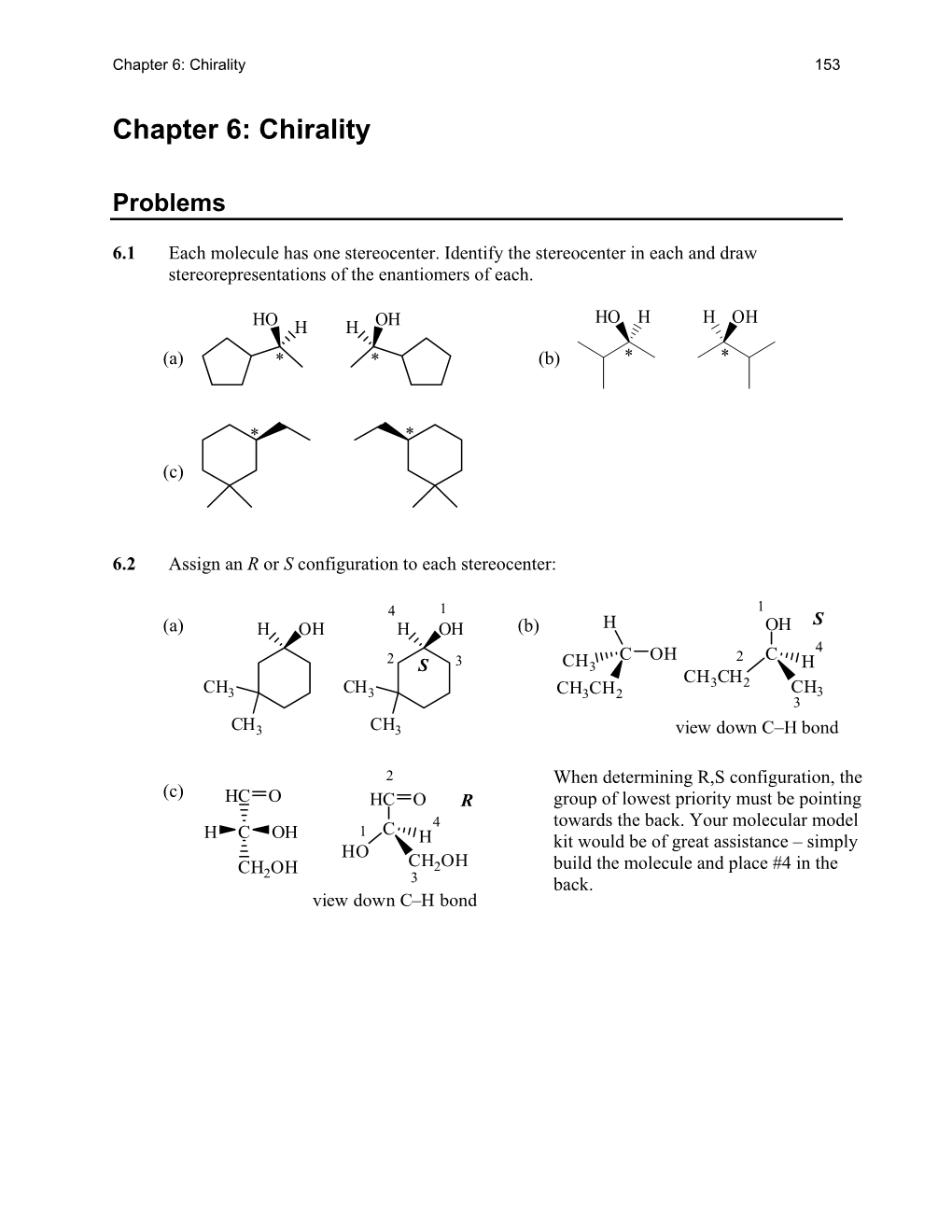
Chapter 6: Chirality 153
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Synthetic Chemistry and Stereoselective Biotransformations
PP Periodica Polytechnica From Synthetic Chemistry and Chemical Engineering Stereoselective Biotransformations to Enzyme Biochemistry – The Bioorganic Chemistry Group at the Budapest 59(1), pp. 59-71, 2015 University of Technology and Economics DOI: 10.3311/PPch.7390 Creative Commons Attribution b Zoltán BOROS1, Gábor HORNYÁNSZKY1, József NAGY1, László POPPE1 * research article Received 04 March 2014; accepted after revision 05 May 2014 Abstract 1 Introduction The activity of Bioorganic Chemistry Group (BCG) within 1.1 Scientific background of the Bioorganic Chemistry Department of Organic Chemistry and Technology at Budapest Research Group University of Technology and Economics is related to various The activity of Bioorganic Chemistry Group (BCG) of areas of synthetic chemistry, biotechnology and enzymology. Department of Organic Chemistry and Technology at Budapest This review gives an overview on the research activity of the University of Technology and Economics is related to various group covering development of synthetic organic chemistry areas of synthetic chemistry, selective biocatalysis [1] and methods; stereoselective biotransformations with lipases, enzymology with major emphasis on development of novel ammonia-lyases and further biocatalysts in batch and tools for stereoselective synthesis [2]. continuous-flow reactions; novel enzyme immobilization One of the main challenges facing organic chemistry is methods; and enzyme structural and mechanistic studies by the rational synthesis of an ever growing number of complex, experimental and computational techniques. optically active natural products and their analogues [3]. According to the regulation of FDA production of chiral Keywords drugs, agrochemicals, fine chemicals has been allowed in synthetic organic chemistry, stereoselective biotransformation, enantiomerically pure form, because it often happens that only continuous-flow reaction, lipase, ammonia-lyase, enzyme one of the two enantiomers shows the required therapeutical immobilization, enzyme structure, enzyme mechanism, QM/ effect [4]. -

Separation of the Mixtures of Chiral Compounds by Crystallization
1 Separation of the Mixtures of Chiral Compounds by Crystallization Emese Pálovics2, Ferenc Faigl1,2 and Elemér Fogassy1* 1Department of Organic Chemistry and Technology, Budapest University of Technology and Economics, 2Research Group for Organic Chemical Technology, Hungarian Academy of Sciences, Budapest, Hungary 1. Introduction Reaction of a racemic acid or base with an optically active base or acid gives a pair of diastereomeric salts. Members of this pair exhibit different physicochemical properties (e.g., solubility, melting point, boiling point, adsorbtion, phase distribution) and can be separated owing to these differences. The most important method for the separation of enantiomers is the crystallization. This is the subject of this chapter. Preparation of enantiopure (ee~100%) compounds is one of the most important aims both for industrial practice and research. Actually, the resolution of racemic compounds (1:1 mixture of molecules having mirror-imagine relationship) still remains the most common method for producing pure enantiomers on a large scale. In these cases the enantiomeric mixtures or a sort of their derivatives are separated directly. This separation is based on the fact that the enantiomeric ratio in the crystallized phase differs from the initial composition. In this way, obtaining pure enantiomers requires one or more recrystallizations. (Figure 1). The results of these crystallizations (recrystallizations) of mixtures of chiral compounds differ from those observed at the achiral compounds. Expectedly, not only the stereoisomer in excess can be crystallized, because the mixture of enantiomers (with mirror image relationship) follows the regularities established from binary melting point phase diagrams, and ternary composition solubility diagrams, respectively, as a function of the starting enantiomer proportion. -

Diastereomers Diastereomers
Diastereomers Diastereomers: Stereoisomers that are not mirror images. enantiomer (R) (S) (S) (R) diastereomers diastereomer diastereomer enantiomer (R) (S) (R) (S) Diastereomers Diastereomers: Stereoisomers that are not mirror images. (R) enantiomer (S) (S) (R) diastereomer To draw the enantiomer of a molecule with chiral centers, invert stereochemistry at all chiral centers. (R) To draw a diastereomer of a molecule (R) with chiral centers, invert stereochemistry at only some chiral centers. Meso Compounds Meso: A molecule that contains chiral centers, but is achiral. 3 Are these molecules chiral? (R) (S) (These are diff eren t f rom th e 3 molecules I just showed; they have 2 -Cl’s, rather than 1 -Cl & 1 -OH. enantiomer (R) (S) (R) (S) These molecules are chiral mirror images of one another. (R,R) and (S,S) are not the same. Meso Compounds Meso: A molecule that contains chiral centers, but is achiral. 3 enantiomer ? (R) (S) (S) (R) no! 3 same molecule! enantiomer (R) (S) (R) (S) Meso Compounds Meso: A molecule that contains chiral centers, but is achiral. 3 enantiomer ? (R) (S) (S) (R) no! 3 same molecule! If a molecule • contains the same number of (R) and (S) stereocenters, and • those stereocenters have identical groups attached, then the molecule is achiral and meso. Meso Compounds Meso: A molecule that contains chiral centers, but is achiral. 3 same molecule (R) (S) (S) (R) 3 meso diastereomers meso diastereomer diastereomer enantiomer (R) (S) (R) (S) chiral chiral Properties of Enantiomers Most physical properties of enantiomers are identical. diethyl-(R,R)-tartrate diethyl-(S,S)-tartrate boiling point 280 °C 280 °C melting point 19 °C 19 °C density 1.204 g/mL 1.204 g/mL refractive index 1.447 1.447 i.e., chirality does not affect most physical properties. -

Chapter 4: Stereochemistry Introduction to Stereochemistry
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane Me Me Me C Me H Bu Bu Me Me 2-methylhexane H H mirror Me rotate Bu Me H 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane H C Et Et Me Pr Pr 3-methylhexane Me Me H H mirror Et rotate H Me Pr 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane .Compounds that are not superimposable with their mirror image are called chiral (in Greek, chiral means "handed") 3-methylhexane is a chiral molecule. .Compounds that are superimposable with their mirror image are called achiral. 2-methylhexane is an achiral molecule. .An atom (usually carbon) with 4 different substituents is called a stereogenic center or stereocenter. Enantiomers Et Et Pr Pr Me CH3 Me H H 3-methylhexane mirror enantiomers Et Et Pr Pr Me Me Me H H Me H H Two compounds that are non-superimposable mirror images (the two "hands") are called enantiomers. Introduction To Stereochemistry Structural (constitutional) Isomers - Compounds of the same molecular formula with different connectivity (structure, constitution) 2-methylpentane 3-methylpentane Conformational Isomers - Compounds of the same structure that differ in rotation around one or more single bonds Me Me H H H Me H H H H Me H Configurational Isomers or Stereoisomers - Compounds of the same structure that differ in one or more aspects of stereochemistry (how groups are oriented in space - enantiomers or diastereomers) We need a a way to describe the stereochemistry! Me H H Me 3-methylhexane 3-methylhexane The CIP System Revisited 1. -

Formation and Crystallization Based Separation of Diastereomeric Salts
Formation and Crystallization based Separation of Diastereomeric Salts Der Fakultät für Verfahrens- und Systemtechnik der Otto-von-Guericke-Universität Magdeburg zur Erlangung des akademischen Grades Doktoringenieur (Dr.-Ing.) am: 03.05.2012. vorgelegte Dissertation (Einreichungsdatum) von: M.Sc. Venkata Subbarayudu Sistla i Schriftliche Erklärung Ich erkläre hiermit, dass ich die vorliegende Arbeit ohne unzulässige Hilfe Dritter und ohne Benutzung anderer als der angegebenen Hilfsmittel angefertigt habe. Die aus fremden Quellen direkt oder indirekt übernommenen Gedanken sind als solche kenntlich gemacht. Insbesondere habe ich nicht die Hilfe einer Kommerziellen Promotionsberatung in Anspruch genommen. Dritte haben von mir weder unmittelbar noch mittelbar geldwerte Leistungen für Arbeiten erhalten, die im Zusammenhang mit dem Inhalt der vorgelegten Dissertation stehen. Die Arbeit wurde bisher weder im Inland noch im Ausland in gleicher oder ähnlicher Form als Dissertation eingereicht und ist als Ganzes auch noch nicht veröffentlicht. (Magdeburg, 03.05.2012) (M.Sc. Venkata Subbarayudu, Sistla) ii It’s an immense pleasure for me to dedicate this work to my Uncle M.K. Ramatarakam garu and to my parents Sistla. Lakshmi Savitri Annapurna Devi and Sistla.Venkateswarlu. iii Acknowledgement First of all, I bow in front of the lord Sita-Rama, Who is there along with me all along in my life and made me to follow the path of truth in all the situations when my mind was not stable. I would like to convey my profound gratitude to Professor Andreas Seidel-Morgenstern and apl. Professor Heike Lorenz as they gave me this great opportunity to explore myself and in the area of Crystallization. I am proud to be in the group PCG under the guidance of Prof. -

Organic & Biomolecular Chemistry
Organic & Biomolecular Chemistry View Article Online REVIEW View Journal | View Issue Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review Cite this: Org. Biomol. Chem., 2016, 14, 6375 Paula Bracco,†a Hanna Busch,†a Jan von Langermannb and Ulf Hanefeld*a The first enantioselective synthesis was the selective addition of cyanide to benzaldehyde catalysed by a hydroxynitrile lyase (HNL). Since then these enzymes have been developed into a reliable tool in organic synthesis. HNLs to prepare either the (R)- or the (S)-enantiomer of the desired cyanohydrin are available and a wide variety of reaction conditions can be applied. As a result of this, numerous applications Received 29th April 2016, of these enzymes in organic synthesis have been described. Here the examples of the last decade are Accepted 31st May 2016 summarised, the enzyme catalysed step is discussed and the follow-up chemistry is shown. This proves DOI: 10.1039/c6ob00934d HNLs to be part of main stream organic synthesis. Additionally the newest approaches via immobilisation www.rsc.org/obc and reaction engineering are introduced. Creative Commons Attribution 3.0 Unported Licence. 1. Introduction they are readily converted to yield α-hydroxy aldehydes or ketones, β-amino alcohols or α-fluorocyanides and many other Enantiopure cyanohydrins are valuable and versatile building compounds. They are therefore of central importance both for blocks in organic chemistry. With their two functional groups the synthesis of fine and bulk chemicals.1 Consequently they are a mainstay in academic and industrial research. The synthesis of enantiopure cyanohydrins is performed by aGebouw voor Scheikunde, Biokatalyse, Afdeling Biotechnologie, the addition of nucleophilic cyanide to a prochiral carbonyl This article is licensed under a Technische Universiteit Delft, Julianalaan 136, 2628BL Delft, The Netherlands. -

A Tool for Chirality Control in Asymmetric Catalysis
Flexibility – A Tool for Chirality Control in Asymmetric Catalysis Raivis Žalubovskis Doctoral Thesis Stockholm 2006 Akademisk avhandling som med tillstånd av Kungl Tekniska Högskolan i Stockholm framlägges till offentlig granskning för avläggande av filosofie doktorsexamen i kemi med inrikting mot organisk kemi, måndagen den 27 november, kl 10.00 i sal F3, KTH, Lindstedtsvägen 26, Stockholm. Avhandlingen försvaras på engelska. Opponent är Professor Alexandre Alexakis, Université de Genève, Schweiz. ISBN 91-7178-491-8 ISRN KTH/IOK/FR--06/104--SE ISSN 1100-7974 TRITA-IOK Forskningsrapport 2006:104 © Raivis Zalubovskis Universitetsservice US AB, Stockholm Zalubovskis, R. 2006 ”Flexibility – A Tool for Chirality Control in Asymmetric Catalysis”, Organic Chemistry, KTH Chemical Science and Engineering, SE-100 44 Stockholm, Sweden. Abstract This thesis deals with the design and synthesis of ligands for asymmetric catalysis: palladium catalyzed allylic alkylations, and rho- dium and iridium catalyzed hydrogenations of olefins. Chirally flexible phosphepine ligands based on biphenyl were synthesized and their properties were studied. The rotation barrier for configurationally flexible phosphepines was determined by NMR spectroscopy. The ratio of the atropisomers was shown to depend on the group bound to phosphorus. Only complexes with two homochiral ligands bound to the metal center were observed upon complexation with Rh(I). It was shown that one diastereomer of the flexible ligand exhibits higher activity but lower selectivity than its diastereomer in the rhodium catalyzed hydrogenation of methyl α-acetamido- cinnamate. These ligands were also tested in nickel catalyzed silabora- tions. Chiral P,N-ligands with pseudo-C2 and pseudo-CS symmetry based on pyrrolidines-phospholanes or azepines-phosphepines were synthesized and studied in palladium catalyzed allylic alkylations. -

Biotechnology Letters 2009 Section: Microbial and Enzyme Technology
Biotechnology Letters 2009 Section: Microbial and Enzyme Technology Review Advances in Enzyme Immobilisation Dean Brady · Justin Jordaan D. Brady Enzyme Technologies, CSIR Biosciences, Ardeer Road, Modderfontein, Private Bag X2, 1645, South Africa. Department of Biotechnology and Food Technology, Tshwane University of Technology, Nelson Mandela Drive, Arcadia Campus, Private Bag X680, Pretoria. 0001. e-mail [email protected] J. Jordaan Molecular Biomaterials, CSIR Biosciences, Meiring Naude Road, Brummeria, 0091, South Africa. 1 Abstract Improvements in current carrier-based immobilisation strategies have been developed using hetero-functionalised supports that enhance the binding efficacy and stability through multipoint attachment. New commercial resins (Sepabeads®) exhibit improved protein binding capacity. Novel methods of enzyme self immobilisation have been developed (CLEC, CLEA, Spherezyme), as well as carrier materials (Dendrispheres), encapsulation (PEI Microspheres), and entrapment. Apart from retention, recovery and stabilisation, other advantages to enzyme immobilisation have emerged, such as enhanced enzyme activity, modification of substrate selectivity and enantioselectivity, and multi-enzyme reactions. These advances promise to enhance the roles of immobilisation enzymes in industry, while opening the door for novel applications. Keywords Biocatalyst, biocatalysis, enzyme, immobilisation, immobilization, Received: 31 March 2009/Accepted: ………./Published: ….. © Springer Science+Business Media B.V. …… 2 Introduction Biocatalytic process economics can be enhanced by enzyme reuse and the improvement in enzyme stability afforded by immobilisation. The capacity to retain or recover enzymes also allows biocatalyst separation from product, thereby permitting continuous processes, and prevents carry-through of protein or activity to subsequent process steps (Polizzi et al. 2007). Immobilisation can also improve enzyme performance under optimal process reaction conditions (e.g. -

Diastereomer
Diastereomer Diastereomers (sometimes called diastereoisomers) are a type of a stereoisomer.[1] Diastereomers are Diastereomers that are also epimers defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other.[2] When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another.[3] Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their D-Threose D-Erythrose mirror image (that is, excluding the opposing enantiomer). Diastereomers have different physical properties (unlike most aspects of enantiomers) and often different chemical reactivity. Diastereomerism can also occur at a double bond, where the cis vs trans relative positions of substituents give two non-superposable isomers. Many conformational isomers are diastereomers as well. Diastereoselectivity is the preference for the formation of one or more than one diastereomer over the other in an organic reaction. Contents Syn / anti Erythro / threo Multiple stereocenters Applications See also References Syn / anti When the single bond between the two centres is free to rotate, cis/trans descriptors become invalid. Two widely accepted prefixes used to distinguish diastereomers on sp³-hybridised bonds in an open-chain molecule are syn and anti. -

Biocatalysis
Biocatalysis • General Principles – Stereoselectivity – Biocatalyst production – Biocatalyst immobilization – Biocatalyst modificatiln • Hydrolytic reactions • Redox reactions • Addition-/elimination reactions • Glycosyl Transfer • Industrial applications • Cascade processes Biocatalysis – General Aspects Stereochemistry & Drug Synthesis • Enantiomers & Diastereomer Discrimination Biocatalysis – General Aspects Stereochemistry & Drug Synthesis • Enantiomers & Diastereomer Discrimination Biocatalysis – General Aspects Stereochemistry & Drug Synthesis • The Thalidomid Incident O O O O H H N N (R) (S) N O N O H H O O Thalidomid: (R)-enantiomer: weak analgetic (S)-enantiomer: strong teratogenic side effects Biocatalysis – General Aspects Pros â high enantioselectivity â high regioselectivity (incl. diastereoselectivity) â high chemoselectivity â broad substrate tolerance â high efficiency â environmentally benign â mild reaction conditions â enzyme compatibility (reaction cascades) Cons â enantiocomplementarity â cofactors â low flexibility in operational parameters â aqueous reaction conditions (loss of activity in organic solvents) â inhibition â availability Biocatalysis – General Aspects Enzymes & Transformations Biocatalysis – General Aspects Induced-Fit-Theory • Koshland 1961 – conformational influence by substrate & enzyme ß modification of the biological activity of proteins lock & key induced fit active center active center Biocatalysis – General Aspects Enantioselectivity • Three-Point Attachment Theory (Ogston 1948) D D chemical -

Resolution of Enantiomers with Both Achiral Phases in Chromatography
RSC Advances View Article Online PAPER View Journal | View Issue Resolution of enantiomers with both achiral phases in chromatography: conceptual challenge Cite this: RSC Adv.,2015,5,28316 a a b Ravi Bhushan,* Hariom Nagar and Jurgen¨ Martens A new practical approach has been adopted for the resolution (from racemic and non-racemic mixtures) of DL-selenomethionine using achiral phase chromatography supplemented with (À)-quinine as a chiral inducing reagent (CIR) and for the resolution of three racemic b-blockers (orciprenaline, betaxolol and atenolol) using L-Glutamic acid as a CIR. These analytes were pre-mixed with CIR and were applied to plain silica gel plates. The chromatograms were developed with the mobile phase having no chiral additive. Investigations were carried out by varying the pH, temperature and ratio of CIR to analytes. Native enantiomers were isolated and the detection limit for each enantiomer of SeMet was found to be Received 25th January 2015 À À 0.18 mgmL 1 and for enantiomers of the b-blockers it was found to be in the range of 1.2–1.8 mgmL 1. Accepted 12th March 2015 Optimized structures of transient diastereomers based on density functional theory using the Gaussian DOI: 10.1039/c5ra01496d 09 Rev. A.02 program and hybrid density functional B3LYP with 6-31G* basis set have been developed. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. www.rsc.org/advances The separation mechanism has also been explained. 1. Introduction achiral phases were presented as a short review by Martens and Bhushan.2 Since then there appeared a few more reports on As per IUPAC,1 the term ‘resolution’ in stereochemistry stands enantioresolution of certain non racemic mixtures in achiral for a process, for the separation of racemic compounds (equi- environment, these included some antihistamines,3 binaph- molar mixture of a pair of enantiomers) into their enantiomers. -

Bringing Biocatalysis Into the Deuteration Toolbox
Bringing biocatalysis into the deuteration toolbox J. S. Rowbotham,1 O. Lenz,2 H. A. Reeve1* and K. A. Vincent1* 1Department of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR, United Kingdom 2Department of Chemistry, Technische Universität Berlin, Strasse des 17. Juni 135, 10623 Berlin, Germany *Correspondence to: [email protected], [email protected] Chemicals labelled with the heavy hydrogen isotope deuterium (2H) have long been used in chemical and biochemical mechanistic studies, spectroscopy, and as analytical tracers.1 More recently, demonstration of selectively deuterated drug candidates that exhibit advantageous pharmacological traits has spurred innovations in metal-catalysed 2H insertion at targeted sites,2–5 but asymmetric deuteration remains a key challenge.6–8 Here we demonstrate an easy-to-implement biocatalytic deuteration strategy, achieving 2 high chemo-, enantio- and isotopic selectivity, requiring only H2O (D2O) and unlabelled dihydrogen under ambient conditions. The vast library of enzymes established for NADH-dependent C=O, C=C, and C=N bond reductions9 have yet to appear in the toolbox of commonly employed 2H-labelling techniques due to requirements for suitable deuterated reducing equivalents.10 By facilitating transfer of deuterium atoms from 2 + H2O solvent to NAD , with H2 gas as a clean reductant, we open up biocatalysis for asymmetric reductive deuteration as part of a synthetic pathway or in late stage functionalisation. We demonstrate enantioselective