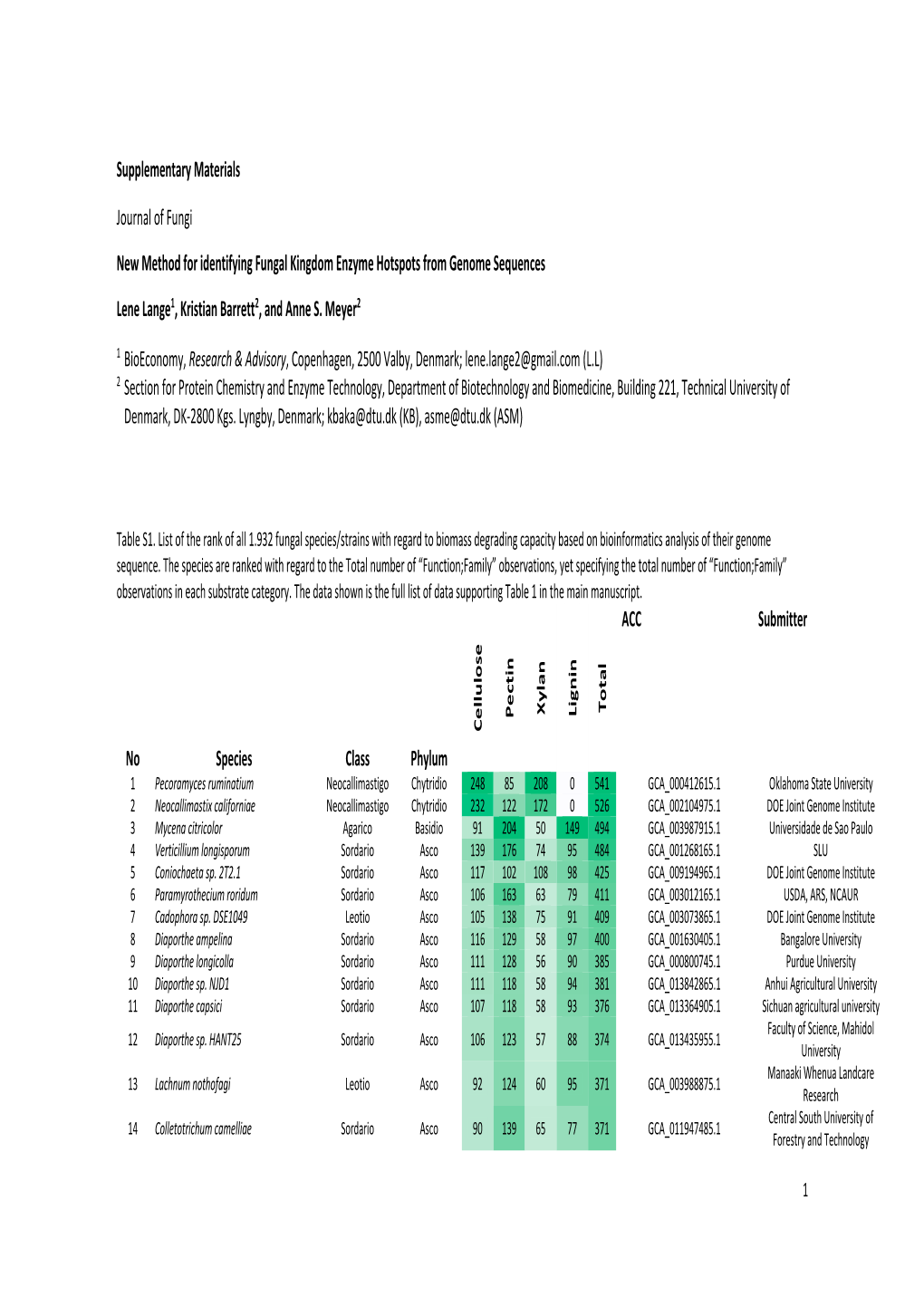
Supplementary Materials Journal of Fungi New Method for Identifying Fungal Kingdom Enzyme Hotspots from Genome Sequences Lene L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity of Endophytic Fungi from Different Verticillium-Wilt-Resistant
J. Microbiol. Biotechnol. (2014), 24(9), 1149–1161 http://dx.doi.org/10.4014/jmb.1402.02035 Research Article Review jmb Diversity of Endophytic Fungi from Different Verticillium-Wilt-Resistant Gossypium hirsutum and Evaluation of Antifungal Activity Against Verticillium dahliae In Vitro Zhi-Fang Li†, Ling-Fei Wang†, Zi-Li Feng, Li-Hong Zhao, Yong-Qiang Shi, and He-Qin Zhu* State Key Laboratory of Cotton Biology, Institute of Cotton Research of Chinese Academy of Agricultural Sciences, Anyang, Henan 455000, P. R. China Received: February 18, 2014 Revised: May 16, 2014 Cotton plants were sampled and ranked according to their resistance to Verticillium wilt. In Accepted: May 16, 2014 total, 642 endophytic fungi isolates representing 27 genera were recovered from Gossypium hirsutum root, stem, and leaf tissues, but were not uniformly distributed. More endophytic fungi appeared in the leaf (391) compared with the root (140) and stem (111) sections. First published online However, no significant difference in the abundance of isolated endophytes was found among May 19, 2014 resistant cotton varieties. Alternaria exhibited the highest colonization frequency (7.9%), *Corresponding author followed by Acremonium (6.6%) and Penicillium (4.8%). Unlike tolerant varieties, resistant and Phone: +86-372-2562280; susceptible ones had similar endophytic fungal population compositions. In three Fax: +86-372-2562280; Verticillium-wilt-resistant cotton varieties, fungal endophytes from the genus Alternaria were E-mail: [email protected] most frequently isolated, followed by Gibberella and Penicillium. The maximum concentration † These authors contributed of dominant endophytic fungi was observed in leaf tissues (0.1797). The evenness of stem equally to this work. -

Genotyping Approach for Potential Common Source of Enterocytozoon
SYNOPSIS Genotyping Approach for Potential Common Source of Enterocytozoon bieneusi Infection in Hematology Unit Guillaume Desoubeaux, Céline Nourrisson, Maxime Moniot, Marie-Alix De Kyvon, Virginie Bonnin, Marjan Ertault De La Bretonniére, Virginie Morange, Éric Bailly, Adrien Lemaignen, Florent Morio, Philippe Poirier Microsporidiosis is a fungal infection that generally causes wide range of host species (1,4). At least 16 microsporidian digestive disorders, especially in immunocompromised species have been described in humans, but Enterocytozoon hosts. Over a 4-day period in January 2018, 3 patients with bieneusi is the most common (5). However, little is known hematologic malignancies who were admitted to the hema- about the actual epidemiology of E. bieneusi microsporidi- tology unit of a hospital in France received diagnoses of En- osis, and there is a need for a better understanding of its terocytozoon bieneusi microsporidiosis. This unusually high pathophysiology and parasitic cycle (3,5). Unfortunately, incidence was investigated by sequence analysis at the in- ternal transcribed spacer rDNA locus and then by 3 micro- epidemiologic studies are complicated because E. bieneusi satellites and 1 minisatellite for multilocus genotyping. The infection has a low incidence rate worldwide (6), and its 3 isolates had many sequence similarities and belonged to microbiological diagnosis is difficult and likely often over- a new genotype closely related to genotype C. In addition, looked (7). In addition, the species is not easy to cultivate multilocus genotyping showed high genetic distances with in vitro in routine practice. Investigations can be carried out all the other strains collected from epidemiologically unre- directly only from infected biologic samples, which usually lated persons; none of these strains belonged to the new use DNA from fecal specimens (1,3,7). -

Distribution of Methionine Sulfoxide Reductases in Fungi and Conservation of the Free- 2 Methionine-R-Sulfoxide Reductase in Multicellular Eukaryotes
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.26.433065; this version posted February 27, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Distribution of methionine sulfoxide reductases in fungi and conservation of the free- 2 methionine-R-sulfoxide reductase in multicellular eukaryotes 3 4 Hayat Hage1, Marie-Noëlle Rosso1, Lionel Tarrago1,* 5 6 From: 1Biodiversité et Biotechnologie Fongiques, UMR1163, INRAE, Aix Marseille Université, 7 Marseille, France. 8 *Correspondence: Lionel Tarrago ([email protected]) 9 10 Running title: Methionine sulfoxide reductases in fungi 11 12 Keywords: fungi, genome, horizontal gene transfer, methionine sulfoxide, methionine sulfoxide 13 reductase, protein oxidation, thiol oxidoreductase. 14 15 Highlights: 16 • Free and protein-bound methionine can be oxidized into methionine sulfoxide (MetO). 17 • Methionine sulfoxide reductases (Msr) reduce MetO in most organisms. 18 • Sequence characterization and phylogenomics revealed strong conservation of Msr in fungi. 19 • fRMsr is widely conserved in unicellular and multicellular fungi. 20 • Some msr genes were acquired from bacteria via horizontal gene transfers. 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.26.433065; this version posted February 27, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

Filling Gaps in the Microsporidian Tree: Rdna Phylogeny of Chytridiopsis Typographi (Microsporidia: Chytridiopsida)
Parasitology Research (2019) 118:169–180 https://doi.org/10.1007/s00436-018-6130-1 GENETICS, EVOLUTION, AND PHYLOGENY - ORIGINAL PAPER Filling gaps in the microsporidian tree: rDNA phylogeny of Chytridiopsis typographi (Microsporidia: Chytridiopsida) Daniele Corsaro1 & Claudia Wylezich2 & Danielle Venditti1 & Rolf Michel3 & Julia Walochnik4 & Rudolf Wegensteiner5 Received: 7 August 2018 /Accepted: 23 October 2018 /Published online: 12 November 2018 # Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Microsporidia are intracellular eukaryotic parasites of animals, characterized by unusual morphological and genetic features. They can be divided in three main groups, the classical microsporidians presenting all the features of the phylum and two putative primitive groups, the chytridiopsids and metchnikovellids. Microsporidia originated from microsporidia-like organisms belong- ing to a lineage of chytrid-like endoparasites basal or sister to the Fungi. Genetic and genomic data are available for all members, except chytridiopsids. Herein, we filled this gap by obtaining the rDNA sequence (SSU-ITS-partial LSU) of Chytridiopsis typographi (Chytridiopsida), a parasite of bark beetles. Our rDNA molecular phylogenies indicate that Chytridiopsis branches earlier than metchnikovellids, commonly thought ancestral, forming the more basal lineage of the Microsporidia. Furthermore, our structural analyses showed that only classical microsporidians present 16S-like SSU rRNA and 5.8S/LSU rRNA gene fusion, whereas the standard eukaryote rRNA gene structure, although slightly reduced, is still preserved in the primitive microsporidians, including 18S-like SSU rRNA with conserved core helices, and ITS2-like separating 5.8S from LSU. Overall, our results are consistent with the scenario of an evolution from microsporidia-like rozellids to microsporidians, however suggesting for metchnikovellids a derived position, probably related to marine transition and adaptation to hyperparasitism. -

How Transposons Drive Evolution of Virulence in a Fungal Pathogen
bioRxiv preprint doi: https://doi.org/10.1101/038315; this version posted January 30, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 How transposons drive evolution of virulence in a fungal pathogen 2 Luigi Faino1#, Michael F Seidl1#, Xiaoqian Shi-Kunne1, Marc Pauper1, Grardy CM van den 3 Berg1, Alexander HJ Wittenberg2, and Bart PHJ Thomma1* 4 5 1Laboratory of Phytopathology, Wageningen University, Droevendaalsesteeg 1, 6708 PB 6 Wageningen, The Netherlands 7 2Keygene N.V., Agro Business Park 90, 6708 PW Wageningen, The Netherlands 8 9 #These authors contributed equally to this work 10 *Corresponding author: Prof. dr. Bart PHJ Thomma 11 Chair, Laboratory of Phytopathology 12 Wageningen University 13 Droevendaalsesteeg 1 14 6708 PB Wageningen 15 The Netherlands 16 Email: [email protected] 17 18 19 Running title: Genome evolution by transposable elements 20 Keywords: Genome evolution; Transposable element; Plant pathogen; Verticillium dahliae; 21 segmental genome duplication 1 bioRxiv preprint doi: https://doi.org/10.1101/038315; this version posted January 30, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 22 Abstract 23 Genomic plasticity enables adaptation to changing environments, which is especially relevant 24 for pathogens that engage in arms races with their hosts. In many pathogens, genes 25 mediating aggressiveness cluster in highly variable, transposon-rich, physically distinct 26 genomic compartments. However, understanding of the evolution of these compartments, 27 and the role of transposons therein, remains limited. -

Secreted Proteins in Microsporidian Parasites: a Functional and Evolutionary Perspective on Host-Parasite Interactions
Secreted proteins in microsporidian parasites: a functional and evolutionary perspective on host-parasite interactions. Submitted by Scott Edward Campbell to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological Science. In September 2013 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from this thesis may be published without proper acknowledgment. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Signature ……………………………………. Page| 1 Abstract The Microsporidia form a phylum of obligate intracellular parasites known to cause disease in humans and a diverse range of economically important animal species. Once classified as ‘primitive’ eukaryotes, it is now recognised that the peculiarities of microsporidian genomics and cell biology are, in fact, the consequence of extreme reduction allowed by an intimate relationship with the host cell. Excluding survival as an extracellular spore, microsporidia are in direct contact with the host throughout their developmental lifecycle, from entry to egress. Host cell manipulations have been described in morphological terms, but despite this, characterisation of such processes at the molecular level remains challenging. The logistics of the microsporidian lifecycle suggest secreted proteins and membrane proteins with extracellular domains may be involved in virulence and implicated in host cell manipulation. This study employs bioinformatic tools to predict secreted proteins in diverse microsporidia and comparative genomics to identify conserved proteins which may be required for host cell manipulation, pathogenicity and lifecycle progression. -

Alternatives in Molecular Diagnostics of Encephalitozoon and Enterocytozoon Infections
Journal of Fungi Review Alternatives in Molecular Diagnostics of Encephalitozoon and Enterocytozoon Infections Alexandra Valenˇcáková * and Monika Suˇcik Department of Biology and Genetics, University of Veterinary Medicine and Pharmacy, Komenského 73, 04181 Košice, Slovakia; [email protected] * Correspondence: [email protected] Received: 15 June 2020; Accepted: 20 July 2020; Published: 22 July 2020 Abstract: Microsporidia are obligate intracellular pathogens that are currently considered to be most directly aligned with fungi. These fungal-related microbes cause infections in every major group of animals, both vertebrate and invertebrate, and more recently, because of AIDS, they have been identified as significant opportunistic parasites in man. The Microsporidia are ubiquitous parasites in the animal kingdom but, until recently, they have maintained relative anonymity because of the specialized nature of pathology researchers. Diagnosis of microsporidia infection from stool examination is possible and has replaced biopsy as the initial diagnostic procedure in many laboratories. These staining techniques can be difficult, however, due to the small size of the spores. The specific identification of microsporidian species has classically depended on ultrastructural examination. With the cloning of the rRNA genes from the human pathogenic microsporidia it has been possible to apply polymerase chain reaction (PCR) techniques for the diagnosis of microsporidial infection at the species and genotype level. The absence of genetic techniques for manipulating microsporidia and their complicated diagnosis hampered research. This study should provide basic insights into the development of diagnostics and the pitfalls of molecular identification of these ubiquitous intracellular pathogens that can be integrated into studies aimed at treating or controlling microsporidiosis. Keywords: Encephalitozoon spp.; Enterocytozoonbieneusi; diagnosis; molecular diagnosis; primers 1. -

Epidemiological, Clinical and Diagnostic Aspects of Sheep Conidiobolomycosis in Brazil
Ciência Rural, Santa Maria,Epidemiological, v.46, n.5, p.839-846, clinical mai, and 2016 diagnostic aspects of sheep conidiobolomycosis http://dx.doi.org/10.1590/0103-8478cr20150935 in Brazil. 839 ISSN 1678-4596 MICROBIOLOGY Epidemiological, clinical and diagnostic aspects of sheep conidiobolomycosis in Brazil Aspectos epidemiológicos, clínicos e de diagnóstico da conidiobolomicose ovina no Brasil Carla WeiblenI Daniela Isabel Brayer PereiraII Valéria DutraIII Isabela de GodoyIII Luciano NakazatoIII Luís Antonio SangioniI Janio Morais SanturioIV Sônia de Avila BottonI* — REVIEW — ABSTRACT As lesões da conidiobolomicose normalmente são de caráter granulomatoso e necrótico, apresentando-se sob duas formas Conidiobolomycosis is an emerging disease caused clínicas: rinofacial e nasofaríngea. O presente artigo tem como by fungi of the cosmopolitan genus Conidiobolus. Particular objetivo revisar as principais características da doença em ovinos, strains of Conidiobolus coronatus, Conidiobolus incongruus and particularizando a epidemiologia, assim como os aspectos clínicos Conidiobolus lamprauges, mainly from tropical or sub-tropical e o diagnóstico das infecções causadas por Conidiobolus spp. no origin, cause the mycosis in humans and animals, domestic or Brasil. Neste País, a enfermidade é endêmica nas regiões nordeste wild. Lesions are usually granulomatous and necrotic in character, e centro-oeste, afetando ovinos predominantemente de raças presenting two clinical forms: rhinofacial and nasopharyngeal. deslanadas, ocasionando a morte na grande maioria dos casos This review includes the main features of the disease in sheep, with estudados. As espécies do fungo responsáveis pelas infecções an emphasis on the epidemiology, clinical aspects, and diagnosis em ovinos são C. coronatus e C. lamprauges e a forma clínica of infections caused by Conidiobolus spp. -

Paula Cristina Azevedo Rodrigues S L T I F U N O N R T O I S P T
Universidade do Minho Escola de Engenharia m o f r o f e Paula Cristina Azevedo Rodrigues s l t i f u n o n r t o i s p t e a c i h s i c n l e a d i g n i c r x a e o s t m d a l n f m o a o c m d l Mycobiota and aflatoxigenic profile of n o a t a e n a s t o Portuguese almonds and chestnuts from e i o t u i c g b u u o production to commercialisation t d c r o y o r M P p s e u g i r d o R o d e v e z A a n i t s i r C a l u a P 0 1 0 2 | o h n i M U November 2010 Universidade do Minho Escola de Engenharia Paula Cristina Azevedo Rodrigues Mycobiota and aflatoxigenic profile of Portuguese almonds and chestnuts from production to commercialisation Dissertation for PhD degree in Chemical and Biological Engineering Supervisors Professor Doutor Nelson Lima Doutor Armando Venâncio November 2010 The integral reproduction of this thesis or parts thereof is authorized only for research purposes provided a written declaration for permission of use Universidade do Minho, November 2010 Assinatura: THIS THESIS WAS PARTIALLY SUPPORTED BY FUNDAÇÃO PARA A CIÊNCIA E A TECNOLOGIA AND THE EUROPEAN SOCIAL FUND THROUGH THE GRANT REF . SFRH/BD/28332/2006, AND BY FUNDAÇÃO PARA A CIÊNCIA E A TECNOLOGIA AND POLYTECHNIC INSTITUTE OF BRAGANÇA THROUGH THE GRANT REF . -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

<I>Neolecta Vitellina
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/118.197 Volume 118, pp. 197–201 October–December 2011 Neolecta vitellina, first record from Romania, with notes on habitat and phenology Vasilică Claudiu Chinan 1* & David Hewitt 2 1Faculty of Biology, Alexandru Ioan Cuza University, Bd. Carol I, No. 20A, 700505, Iaşi, Romania 2Department of Botany, Academy of Natural Sciences 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103 USA * Correspondence to: [email protected] Abstract — Neolecta vitellina, one of the rarely collected ascomycete species in Europe, is reported from Romania for the first time. The species was found on the ground, under Norway spruce (Picea abies) in 2004, from the Nature Reserve “Tinovul Mare” Poiana Stampei (Eastern Carpathians, Romania). Subsequent field observations have confirmed the presence of Neolecta vitellina in the same location, in the period 2005–10. A description and photographs of the specimens are presented. Key words — Ascomycota, Neolectaceae, ITS sequence Introduction The genus Neolecta Speg. belongs to the ascomycete family Neolectaceae (Redhead 1977), order Neolectales (Landvik et al. 1993), class Neolectomycetes (Taphrinomycotina, Ascomycota). This genus includes three accepted species —N. flavovirescens Speg., N. irregularis (Peck) Korf & J.K. Rogers, N. vitellina— with clavate, unbranched to lobed yellow ascomata, up to about 7 cm tall (Landvik et al. 2003). Of these three species, only N. vitellina has been reported from Europe (Bresadola 1882, Geitler 1958, Hansen & Knudsen 2000: 48–50, Krieglsteiner 1993: 421, Landvik et al. 2003, Ohenoja 1975, Redhead 1989). Work published by European researchers about Neolecta vitellina refers mainly to phylogeny, morphology and ultrastructure (Landvik 1996, 1998; Landvik et al.