Purdue Pharma Inc
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

XCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) (X) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 ( ) TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 0-19034 REGENERON PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) New York 13-3444607 (State or other jurisdiction of (I.R.S. Employer Identification No) incorporation or organization) 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 (Address of principal executive offices) (Zip code) (914) 347-7000 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None (Title of Class) Securities registered pursuant to Section 12(g) of the Act: Common Stock - par value $.001 per share (Title of Class) Preferred Share Purchase Rights expiring October 18, 2006 (Title of Class) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes /X/ No Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (ss. 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -

Virginia: in the Circuit Court of Pittsylvania County
VIRGINIA: IN THE CIRCUIT COURT OF PITTSYLVANIA COUNTY PITTSYLVANIA COUNTY, Plaintiff, v. PURDUE PHARMA, L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; RHODES PHARMACEUTICALS, L.P.; ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC.; MALLINCKRODT PLC; MALLINCKRODT LLC; ENDO HEALTH SOLUTIONS, INC; ENDO PHARMACEUTICALS, INC.; PAR Case No. CL18 - __________ PHARMACEUTICAL COMPANIES, INC.; PAR PHARMACEUTICAL, INC.; TEVA Jury Trial Demanded PHARMACEUTICALS USA, INC.; CEPHALON, INC.; BARR LABORATORIES, INC.; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC.; WATSON LABORATORIES, INC.; ALLERGAN PLC; ACTAVIS PHARMA, INC.; ACTAVIS, LLC; INSYS THERAPEUTICS, INC.; KVK-TECH, INC.; AMNEAL PHARMACEUTICALS LLC; IMPAX LABORATORIES, LLC; AMNEAL PHARMACEUTICALS, INC.; AMNEAL PHARMACEUTICALS OF NEW YORK, LLC; MYLAN PHARMACEUTICALS, INC.; MCKESSON CORPORATION; MCKESSON MEDICAL-SURGICAL INC.; CARDINAL HEALTH, INC.; AMERISOURCEBERGEN DRUG CORPORATION; HENRY SCHEIN, INC.; GENERAL INJECTABLES & VACCINES, INC.; INSOURCE, INC.; CVS HEALTH CORPORATION; CVS PHARMACY, INC.; CVS TN DISTRIBUTION, L.L.C.; WALGREENS BOOTS ALLIANCE, INC.; WALGREEN CO.; EXPRESS SCRIPTS HOLDING COMPANY; EXPRESS SCRIPTS, INC; CAREMARK RX, L.L.C.; CAREMARKPCS HEALTH, L.L.C.; CAREMARK, L.L.C.; UNITEDHEALTH GROUP INCORPORATED; OPTUM, INC.; OPTUMRX, INC.; and DOES 1-100, Defendants. PLAINTIFF’S ORIGINAL COMPLAINT Plaintiff, Pittsylvania County, Virginia, by and through the undersigned attorneys, (hereinafter “Plaintiff,” “Pittsylvania -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

Complaint, “Chronic Pain” Means Non-Cancer Pain Lasting Three Months Or Longer
TABLE OF CONTENTS Page I. INTRODUCTION ...............................................................................................................1 II. JURISDICTION AND VENUE ..........................................................................................8 III. PARTIES .............................................................................................................................8 A. Plaintiff ....................................................................................................................8 B. Defendants ...............................................................................................................9 IV. FACTUAL ALLEGATIONS ............................................................................................14 A. Defendants Used Multiple Avenues To Disseminate Their False And Deceptive Statements About Opioids. ...................................................................14 1. Defendants spread and continue to spread their false and deceptive statements through direct marketing of their branded opioids. .......................................................................................................15 2. Defendants used a diverse group of seemingly independent third parties to spread false and deceptive statements about the risks and benefits of opioids.................................................................17 a. Key Opinion Leaders (“KOLs”) ....................................................19 (1) Russell Portenoy ................................................................20 -

Non-Merger Civil Enforcement: an Overview of Recent DOJ and FTC Federal Court Litigation
Antitrust , Vol. 32, No. 1, Fall 201 7. © 2017 by the American Bar Association. Reproduced with permission. All rights reserved. This information or any portion thereof may not be copied or disseminated in any form or by any means or stored in an electronic database or retrieval system without the express written consent of the American Bar Association. Non-Merger Civil Enforcement: An Overview of Recent DOJ and FTC Federal Court Litigation BY SONIA KUESTER PFAFFENROTH ECENT YEARS HAVE SEEN THE As a result, there are now a significant number of career attor - Department of Justice and the Federal Trade neys and economists with recent federal trial court experience, Commission appearing with regularity in fed - which they will bring to future cases at the investigative phase eral district court, with the agencies demon - with an eye towards potential litigation. strating a willingness to litigate in both the Rmerger and non-merger context and with a number of high- DOJ Litigation profile trials now in the rearview mirror. Because the DOJ has no administrative adjudicative process, While the majority of civil conduct enforcement actions its civil enforcement cases, whether they are settlements or continue to be filed concurrently with settlements—which contested litigation, are filed directly in federal district court. provide significant insight into the government’s theories— In recent years, the DOJ has litigated a number of cases alleg - both agencies have seen an uptick in the number of contest - ing Section 1 violations and one case alleging a Section 2 vio - ed cases filed in federal district court since the beginning of lation. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

November 26, 2018 Dr. Sol Barer Chairman of the Board Teva
November 26, 2018 Dr. Sol Barer Chairman of the Board Teva Pharmaceutical Industries Limited Basel Street, P.O. Box 3190, Petach Tikva 4951033, Israel Dear Dr. Barer, We write to you as members of the Investors for Opioid Accountability (IOA) which represents a diverse global coalition of public, faith-based, labor, and sustainable funds, as well as asset managers with $2.2 trillion in assets under management.1 The IOA came together in 2017 out of concern for the potential risks that opioids present for the companies in which we invest. As global investors, we are writing to request a meeting with you to discuss potential financial, legal and reputational risks Teva Pharmaceutical Industries Limited (“Teva”) is facing related to the manufacturing and sale of opioids, and to ask that the Board consider adopting governance reforms designed to mitigate those risks. Opioid abuse is undeniably a public health crisis across North America and now spreading outside the U.S. as well. The U.S. Centers for Disease Control and Prevention (“CDC”) report that in 2016 alone, opioid abuse caused 42,249 deaths in the United States, or 115 people per day. In Canada, there were approximately 4,000 opioid-related deaths in 2017, a 34% increase from the prior year and a rate of 10.9 deaths per 100,000 people. In the UK and Wales, people dying from opioids related deaths in 2016 reached a record high of 3,700. In the U.S., the economic and social effects of the opioid crisis have been profound. Economist Alan Krueger has estimated that nearly 50% of prime age non-labor force men take prescription medication on a daily basis, with almost two-thirds of these being prescription pain medication. -

Bio/Pharmaceutical Outsourcing Report: November 2016
Volume 21, Number 11, November 2016 Bio/Pharmaceutical Outsourcing Report Part of PharmSource STRATEGIC ADVANTAGE Business Conditions 1 Business Conditions 1 PE-Owned Companies in Line to Change Hands PE-Owned Companies in Line to Change Hands 2 Commercial Dose Manufacturing and Mergers and acquisitions are a hot topic in the contract services industry these Packaging days, and there is a lot of curiosity about which companies are likely to change 2 New Manufacturing Deal, Temporary hands. Of course, any company is a target at any time, especially when the price is Closures and Recall for Patheon right, but insight to likely acquisition candidates can be gained by looking at the list 3 Commercial Dose Manufacturing and Packaging in Brief 3 Clinical Dose Manufacturing and leastof companies four years owned ago are by likelyprivate targets equity in (PE) the nextfirms. year On oraverage, two. PE firms tend to hold Packaging onto their investments for about five years, so companies that were acquired at 3 Marken to be Acquired by UPS The table on page 2 lists 23 CMC services companies that are PE-owned; the list 5 Clinical Dose Manufacturing and was derived from the PharmSource Strategic Advantage database “Search by Packaging in Brief Ownership” feature. It is sorted by the year each company was acquired, and 6 Side Effects: Impacts of Key Events on suggests that at least 13 CMOs and CDMOs are ripe for a transaction in the foresee- CMOs and CROs able future. 7 API — Large Molecule The sale of one company, Marken (Durham, N.C., USA), was announced just this 7 Samsung BioLogics Completes IPO month (see news item, Page 3); another, Capsugel (Morristown, N.J., USA), has been 8 API — Large Molecule in Brief mentioned in the business press as being prepared for an ownership change. -

Comparison of Oral Contraceptives and Non-Oral Alternatives
PL Detail-Document #290305 −This PL Detail-Document gives subscribers additional insight related to the Recommendations published in− PHARMACIST’S LETTER / PRESCRIBER’S LETTER March 2013 Comparison of Oral Contraceptives and Non-Oral Alternatives —More information about the use of contraceptives is available in our PL Detail-Document, Hormonal Contraception— Productsa Manufacturerb Estrogen Progestin LOW-DOSE MONOPHASIC PILLS Aviane-28 Teva EE 20 mcg Levonorgestrel 0.1 mg Falmina Novast Lessina Teva Lutera Actavis Orsythia Qualitest Sronyx Actavis Gildess Fe 1/20 Qualitest EE 20 mcg Norethindrone acetate Junel 1/20 Teva 1 mg Junel Fe 1/20 Teva Loestrin-21 1/20 Warner Chilcott/Teva Loestrin Fe 1/20 Warner Chilcott/Teva Microgestin 1/20 Actavis Microgestin Fe 1/20 Actavis Generess Fe chewable Actavis EE 25 mcg Norethindrone 0.8 mg Altavera Sandoz EE 30 mcg Levonorgestrel 0.15 mg Kurvelo Lupin Levora Actavis Marlissa Glenmark Nordette-28 Duramed/Teva Portia-28 Teva Cryselle-28 Teva EE 30 mcg Norgestrel 0.3 mg Elinest Novast Low-Ogestrel-21 Actavis Low-Ogestrel-28 Actavis Lo/Ovral-28 Wyeth Gildess Fe 1.5/30 Qualitest EE 30 mcg Norethindrone acetate Junel 1.5/30 Teva 1.5 mg Junel Fe 1.5/30 Teva Loestrin 1.5/30-21 Warner Chilcott/Teva Loestrin Fe 1.5/30 Warner Chilcott/Teva Microgestin 1.5/30 Actavis Microgestin Fe 1.5/30 Actavis More. Copyright © 2013 by Therapeutic Research Center 3120 W. March Lane, Stockton, CA 95219 ~ Phone: 209-472-2240 ~ Fax: 209-472-2249 www.PharmacistsLetter.com ~ www.PrescribersLetter.com ~ www.PharmacyTechniciansLetter.com -
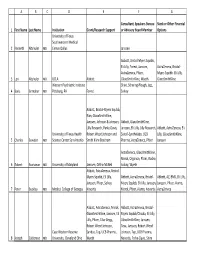
1 2 3 4 5 6 7 8 ABCDEFG First Name Last Name
ABC D E F G Consultant, Speakers Bureau Stock or Other Financial 1 First Name Last Name Institution Grant/Research Support or Advisory Board Member Options University of Texas Southwestern Medical 2 Kenneth Altshuler MD Center Dallas Janssen Abbott, Bristol‐Myers Squibb, Eli Lilly, Forest, Janssen, AstraZeneca, Bristol‐ AstraZeneca, Pfizer, Myers Squibb. Eli Lilly, 3 Lori Altshuler MD UCLA Abbott GlaxoSmithKline, Wyeth GlaxoSmithKline Western Psychiatric Institute Shire, Schering‐Plough, Jazz, 4 Boris Birmaher MD Pittsburg, PA Forest Solvay Abbott, Bristol‐Myers Squibb, Elan, GlaxoSmithKline, Janssen, Johnson & Johnson, Abbott, GlaxoSmithKline, Lilly Research, Parke‐Davis, Janssen, Eli Lilly, Lilly Research, Abbott, AstraZeneca, Eli University of Texas Health Robert Wood Johnson and Sanofi‐Synthelabo, UCB Lilly, GlaxoSmithKline, 5 Charles Bowden MD Science Center San Antonio Smith Kline Beecham Pharma, AstraZeneca, Pfizer Janssen AstraZeneca, GlaxoSmithKline, Merck, Organon, Pfizer, Roche, 6 Robert Buchanan MD University of Maryland Janssen, Ortho‐McNeil Solvay, Wyeth Abbott, AstraZeneca, Bristol‐ Myers Squibb, Eli Lilly, Abbott, AstraZeneca, Bristol‐ Abbott, AZ, BMS, Eli Lilly, Janssen, Pfizer, Solvay, Myers Squibb, Eli Lilly, Janssen, Janssen, Pfizer, Alamo, 7 Peter Buckley MD Medical College of Georgia Novartis Merck, Pfizer, Alamo, Novartis AstraZeneca Abbott, AstraZeneca, Merck, Abbott, AstraZeneca, Bristol‐ GlaxoSmithKline, Janssen, Eli Myers Squibb/Otsuka, Eli Lilly, Lilly, Pfizer, Ciba‐Geigy, GlaxoSmithKline, Janssen, Robert Wood -

Oral Presentation Disclosures
Oral Presentation Disclosures Adler, Lenard – Alcobra Pharma, APSARD/Pond Foundation, Major League Baseball, Major League Baseball Players Association, National Football League, New York University School of Medicine, Novartis Bioventures, Shire Pharmaceuticals, Sunovion, SUNY Upstate, Theravance, US Department of Veterans Affairs Cooperative Studies Program Anton, Raymond – Abbvie, Alkermes, Eli Lilly, Ethypharm, Lundbeck, Pfizer, Sunpharma Baker, Ross – Otsuka Pharmaceutical Development & Commercialization, Inc. Baldwin, David – Lundbeck Beaver, Jessica – Targacept, Inc. Bencherif, Merouane – Targacept, Inc. Bertolino, Alessandro – F. Hoffmann-La Roche, Ltd. Bradshaw, Mark – Euthymics Bioscience, Neurovance, Inc. Burdick, Katherine – Dainippon Sumitomo Pharma Bymaster, Frank – Euthymics Bioscience, Neurovance, Inc. Calabrese, Joseph – Sunovion, Teva (Cephalon) Cantillon, Marc – Forest, Kyowa, Lilly, Merck, Pfizer, Reviva Caroff, Stanley – Sunovion Chen, Yinzhong – Takeda Development Center Americas, Inc. Chengappa, Roy – Pfizer, Inc. Childress, Ann – Abbott Laboratories, Bristol Myer Squibb, GlaxoSmithKline, Ironshore, Janssen (Ortho-McNeil), Johnson & Johnson PRD, Lilly, Neos Therapeutics, Neurovance Inc., NextWave, Novartis, Noven, Otsuka, Pfizer, Rhodes, Sepracor, Shionogi, Shire, Somerset, Sunovion, Theravance Christine, Mazzucco – Janssen Cohen, Lee – Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Inc., GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, Noven Pharmaceuticals, Ortho-McNeil -
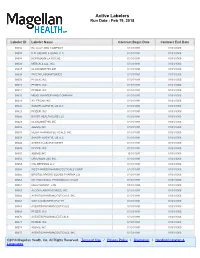
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD.