Published in Acta Palaeontologica Polonica
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Morrison Formation 37 Cretaceous System 48 Cloverly Formation 48 Sykes Mountain Formation 51 Thermopolis Shale 55 Mowry Shale 56
THE STRUCTURAL AND STRATIGRAPHIC FRAMEWORK OF THE WARM SPRINGS RANCH AREA, HOT SPRINGS COUNTY, WYOMING By CHRISTOPHER JAY CARSON Bachelor of Science Oklahoma State University 1998 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 2000 THE STRUCTURAL AND STRATIGRAPHIC FRAMEWORK OF THE WARM SPRINGS RANCH AREA, HOT SPRINGS COUNTY, WYOMING Thesis Approved: Thesis Advisor ~~L. ... ~. ----'-"'-....D~e~e:.-g-e----- II ACKNOWLEDGEMENTS I wish to express appreciation to my advisor Dr. Arthur Cleaves for providing me with the opportunity to compile this thesis, and his help carrying out the fieldwork portion of the thesis. My sincere appreciation is extended to my advisory committee members: Dr. Stan Paxton, Dr. Gary Stewart, and Mr. David Schmude. I wish to thank Mr. Schmude especially for the great deal of personal effort he put forth toward the completion of this thesis. His efforts included financial, and time contributions, along with invaluable injections of enthusiasm, advice, and friendship. I extend my most sincere thank you to Dr. Burkhard Pohl, The Big Hom Basin Foundation, and the Wyoming Dinosaur Center. Without whose input and financial support this thesis would not have been possible. In conjunction I would like to thank the staff of the Wyoming Dinosaur Center for the great deal of help that I received during my stay in Thermopolis. Finally I wish to thank my friends and family. To my friends who have pursued this process before me, and with me; thank you very much. -

Depositional Systems in the Paluxy Formation (Lower Cretaceous), Northeast Texas- Oil,Gas, and Groundwater Resources by Charles A
Geological Circular 77-8 Depositional Systems in the Paluxy Formation (Lower Cretaceous), Northeast Texas- Oil,Gas, and Groundwater Resources BY Charles A. Caughey BUREAU OF ECONOMIC GEOLOGY THE UNIVERSITY OF TEXAS AT AUSTIN AUSTIN, TEXAS 78712 W.L. FISHER, DIRECTOR 1977 Second Printing, 1985 GEOLOGICAL CIRCULAR 77-8 Depositional Systems in thePaluxy Formation (Lower Cretaceous), Northeast Texas- Oil,Gas, and Groundwater Resources BY Charles A. Caughey BUREAU OF ECONOMIC GEOLOGY THE UNIVERSITY OF TEXAS AT AUSTIN AUSTIN, TEXAS 78712 W.L. FISHER, DIRECTOR 1977 SecondPrinting, 1985 Contents Abstract 1 Dispersal patterns 28 Introduction 1 Structural influence 28 Structural framework 3 Resources 28 Stratigraphicrelationships 4 Groundwater 29 Sedimentary facies 5 Outcrop 29 Depositions!systems 8 Subsurface 29 Fluvial system 8 South area 30 Channel-fill and floodbasin facies 9 Meanderbelt facies 11 Central area 31 Fluvial model 12 North area 34 Delta system 13 Conclusions 35 Coastal barrier facies 14 Oil and gas 35 Proximal subfacies 14 Minor producingareas 38 Distal subfacies 16 South Bosque field 38 Lagoon facies 17 Updip trend 38 Deltamodel 19 Major producingareas 41 Classification 19 Fault zone trend 41 Holocene analog 19 Downdipproduction 42 Strandplain system 22 Proximal trend 42 Strandplain facies 22 Distal trend 43 Strandplainmodel 26 Summary 45 Sediment dispersal 27 References 47 Source areas 27 Appendix .. 50 Figures 1. Location map 2 6. Depositional systemsand facies,Paluxy 2. Major structural features of northeast Formationand equivalent lithostrati- Texas 3 graphic units,northeast Texas 7 3. Distribution of Paluxy Formation and 7. Representativeelectric logpatternand related stratigraphic units,surface and shale content of channel sequencesin the subsurface,northeast Texas 4 channel-fill and floodbasin facies,Paluxy fluvial system 9 4. -

Papers in Press
Papers in Press “Papers in Press” includes peer-reviewed, accepted manuscripts of research articles, reviews, and short notes to be published in Paleontological Research. They have not yet been copy edited and/or formatted in the publication style of Paleontological Research. As soon as they are printed, they will be removed from this website. Please note they can be cited using the year of online publication and the DOI, as follows: Humblet, M. and Iryu, Y. 2014: Pleistocene coral assemblages on Irabu-jima, South Ryukyu Islands, Japan. Paleontological Research, doi: 10.2517/2014PR020. doi:10.2517/2018PR013 Features and paleoecological significance of the shark fauna from the Upper Cretaceous Hinoshima Formation, Himenoura Group, Southwest Japan Accepted Naoshi Kitamura 4-8-7 Motoyama, Chuo-ku Kumamoto, Kumamoto 860-0821, Japan (e-mail: [email protected]) Abstract. The shark fauna of the Upper Cretaceous Hinoshima Formation (Santonian: 86.3–83.6 Ma) of the manuscriptHimenoura Group (Kamiamakusa, Kumamoto Prefecture, Kyushu, Japan) was investigated based on fossil shark teeth found at five localities: Himedo Park, Kugushima, Wadanohana, Higashiura, and Kotorigoe. A detailed geological survey and taxonomic analysis was undertaken, and the habitat, depositional environment, and associated mollusks of each locality were considered in the context of previous studies. Twenty-one species, 15 genera, 11 families, and 6 orders of fossil sharks are recognized from the localities. This assemblage is more diverse than has previously been reported for Japan, and Lamniformes and Hexanchiformes were abundant. Three categories of shark fauna are recognized: a coastal region (Himedo Park; probably a breeding site), the coast to the open sea (Kugushima and Wadanohana), and bottom-dwelling or near-seafloor fauna (Kugushima, Wadanohana, Higashiura, and Kotorigoe). -

A New Microvertebrate Assemblage from the Mussentuchit
A new microvertebrate assemblage from the Mussentuchit Member, Cedar Mountain Formation: insights into the paleobiodiversity and paleobiogeography of early Late Cretaceous ecosystems in western North America Haviv M. Avrahami1,2,3, Terry A. Gates1, Andrew B. Heckert3, Peter J. Makovicky4 and Lindsay E. Zanno1,2 1 Department of Biological Sciences, North Carolina State University, Raleigh, NC, USA 2 North Carolina Museum of Natural Sciences, Raleigh, NC, USA 3 Department of Geological and Environmental Sciences, Appalachian State University, Boone, NC, USA 4 Field Museum of Natural History, Chicago, IL, USA ABSTRACT The vertebrate fauna of the Late Cretaceous Mussentuchit Member of the Cedar Mountain Formation has been studied for nearly three decades, yet the fossil-rich unit continues to produce new information about life in western North America approximately 97 million years ago. Here we report on the composition of the Cliffs of Insanity (COI) microvertebrate locality, a newly sampled site containing perhaps one of the densest concentrations of microvertebrate fossils yet discovered in the Mussentuchit Member. The COI locality preserves osteichthyan, lissamphibian, testudinatan, mesoeucrocodylian, dinosaurian, metatherian, and trace fossil remains and is among the most taxonomically rich microvertebrate localities in the Mussentuchit Submitted 30 May 2018 fi fi Accepted 8 October 2018 Member. To better re ne taxonomic identi cations of isolated theropod dinosaur Published 16 November 2018 teeth, we used quantitative analyses of taxonomically comprehensive databases of Corresponding authors theropod tooth measurements, adding new data on theropod tooth morphodiversity in Haviv M. Avrahami, this poorly understood interval. We further provide the first descriptions of [email protected] tyrannosauroid premaxillary teeth and document the earliest North American record of Lindsay E. -

Oldest Known Dinosaurian Nesting Site and Reproductive Biology of the Early Jurassic Sauropodomorph Massospondylus
Oldest known dinosaurian nesting site and reproductive biology of the Early Jurassic sauropodomorph Massospondylus Robert R. Reisza,1, David C. Evansb, Eric M. Robertsc, Hans-Dieter Suesd, and Adam M. Yatese aDepartment of Biology, University of Toronto Mississauga, Mississauga, ON L5L 1C6, Canada; bRoyal Ontario Museum, Toronto, ON M5S 2C6, Canada; cSchool of Earth and Environmental Sciences, James Cook University, Townsville, 4811 QLD, Australia; dDepartment of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013; and eBernard Price Institute for Palaeontological Research, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa Edited by Steven M. Stanley, University of Hawaii, Honolulu, HI, and approved December 16, 2011 (received for review June 10, 2011) The extensive Early Jurassic continental strata of southern Africa clutches and recognition of the earliest known dinosaurian nesting have yielded an exceptional record of dinosaurs that includes complex at the Rooidraai locality. scores of partial to complete skeletons of the sauropodomorph Massospondylus, ranging from embryos to large adults. In 1976 an Results incomplete egg clutch including in ovo embryos of this dinosaur, Our work at the Rooidraai locality has yielded multiple in situ the oldest known example in the fossil record, was collected from clutches of eggs as well as fragmentary eggshell and bones, all from a road-cut talus, but its exact provenance was uncertain. An exca- a 2-m-thick interval of muddy siltstone 25 m from the top of the vation program at the site started in 2006 has yielded multiple in Lower Jurassic Upper Elliot Formation (“Stormberg Group,” situ egg clutches, documenting the oldest known dinosaurian Karoo Supergroup) (11). -

Saurian Safari a Walk in the Jurassic Park
Dinosaur Safari Junior Introduction: The rules are a simplified variant Of the Saurian Safari rules developed by Chris Peers and originally published by HLBS publishing 2002, this an instructional aid used for the Smithsonian Summer camp program. The rules were developed for grades K – 5 but fun with older age groups. The instructor will act as a Game Master (GM) and will be responsible for preparing the character sheets and setting up the map. This custom version is for a fast moving with minimal book keeping or rolling on charts. Game time recommended for 45 – 60 minutes per scenario. A. Preparation: The GM will have printer character sheets and a six-sided die (D6) and a ten-sided die (D10). Each gamer will need a figure and character sheet. A large vinyl hex map crossed with a river is used for the camp. 1. Setup map. The GM will set up the hex map. Terrain types are forest, river or swamp, clear or hills. Follow guidelines for map set up listed for the scenario. The goal is represent the environment of the period. SAURIAN SAFARI JR: CHARACTER SHEET Name: Shooting Skill: (D6x10 + 40) Agility: (D6+ 4) Weapon: Range: Penetration: Damage: Trophies: Species Taken Other Information 2. Help gamers create a character. GM will to help gamers. Calculate the following and record on sheet. For name write gamers name. For Shooting skill roll 1 D6 multiple by 10 and add 40. For Agility roll D6 and add 4. Chose one weapon. Dinosaur Gun. Penetration 12 Damage 8 Nitro Express, double barrel, -5 subtracted from character accuracy. -
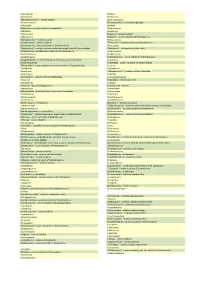
Dino Cards Project D E F List B
Daanosaurus Efraasia Dacentrurus Einiosaurus "Dachongosaurus" – nomen nudum Ekrixinatosaurus Daemonosaurus Elachistosuchus – a rhynchocephalian Dahalokely Elaltitan Dakosaurus – a metriorhynchid crocodilian Elaphrosaurus Dakotadon Elmisaurus Dakotaraptor Elopteryx - nomen dubium Daliansaurus Elosaurus – junior synonym of Brontosaurus "Damalasaurus" – nomen nudum Elrhazosaurus Dandakosaurus - nomen dubium "Elvisaurus" – nomen nudum; Cryolophosaurus Danubiosaurus – junior synonym of Struthiosaurus Emausaurus "Daptosaurus" – nomen nudum; early manuscript name for Deinonychus Embasaurus - theropoda incertae sedis Darwinsaurus - possible junior synonym of Huxleysaurus Enigmosaurus Dashanpusaurus Eoabelisaurus Daspletosaurus Eobrontosaurus – junior synonym of Brontosaurus Dasygnathoides – a non-dinosaurian archosaur, junior synonym Eocarcharia of Ornithosuchus Eoceratops – junior synonym of Chasmosaurus "Dasygnathus" – preoccupied name, now known as Dasygnathoides Eocursor Datanglong Eodromaeus Datonglong "Eohadrosaurus" – nomen nudum; Eolambia Datousaurus Eolambia Daurosaurus – synonym of Kulindadromeus Eomamenchisaurus Daxiatitan Eoplophysis - Dinosauria indet. Deinocheirus Eoraptor Deinodon – possibly Gorgosaurus Eosinopteryx - Avialae Deinonychus Eotrachodon Delapparentia - probable junior synonym of Iguanodon Eotriceratops Deltadromeus Eotyrannus Demandasaurus Eousdryosaurus Denversaurus Epachthosaurus Deuterosaurus – a therapsid Epanterias – may be Allosaurus Diabloceratops "Ephoenosaurus" – nomen nudum; Machimosaurus (a crocodilian) Diamantinasaurus -

Aptian–Albian) of Texas and Oklahoma
Reappraisal of the tribosphenidan mammals from the Trinity Group (Aptian–Albian) of Texas and Oklahoma BRIAN M. DAVIS and RICHARD L. CIFELLI Davis, B.M. and Cifelli, R.L. 2011. Reappraisal of the tribosphenidan mammals from the Trinity Group (Aptian–Albian) of Texas and Oklahoma. Acta Palaeontologica Polonica 56 (3): 441–462. The Trinity therians have long been the focus of attempts to reconstruct the evolutionary history of higher mammals, es− pecially in the context of the development of tribospheny. In this paper, we update the taxonomy of the tribosphenidan taxa known from the Trinity Group and establish with more confidence the premolar/molar count in each. Many isolated specimens can be referred to a specific tooth locus. Additional diversity is revealed within the Deltatheroida, with the de− scription of an additional species of Oklatheridium; Pappotherium is here considered a likely metatherian based on the in− ferred presence of four molars, while Holoclemensia is a basal eutherian (the opposite of some traditional interpretations). The remainder of the genera, Kermackia and Slaughteria, cannot be allied with either of the living groups of tribo− sphenidan mammals using the available data. We identify strong morphological diversity within this assemblage of stem taxa, including modifications to the traditional tribosphenic occlusal pattern in Kermackia. Mammalian evolution at the base of the tribosphenidan radiation was complex, and this underscores the need for caution when interpreting the mor− phology and relationships of taxa known by incomplete material. Key words: Tribosphenida, Metatheria, Eutheria, Deltatheroida, Trinity Group, Early Cretaceous. Brian M. Davis [[email protected]] and Richard L. Cifelli [[email protected]], Department of Zoology and Sam Noble Oklahoma Museum of Natural History, University of Oklahoma, 2401 Chautauqua Ave, Norman, OK, 73072, USA. -

Black Mesa Now Open New Dinosaur Discovered
THE UNIVERSITY OF OKLAHOMA TracksSpring 2011 Newsletter, Volume 23, Number 1 BLACK MESA NOW OPEN Whitten-Newman Foundation Funds Exhibit NEW DINOSAUR DISCOVERED Meet Brontomerus mcintoshi, “Thunder Thighs” ARCHAEOLOGY CURATOR TO RETIRE A Conversation with Don Wyckoff CONTENTS MUSEUM EVENTS AND HIGHLIGHTS DONATION: PAGE 3 EXHIBIT: PAGES 4-5 COLLECTIONS PAGES: 6-7 COMING UP: PAGE 11 NEWS: PAGES: 12-13 features & departments FROM THE DIRECTOR ………… 2 CURATOR ………………………… 8-9 NEWS ……………………………… 12-13 Thanks to Museum Members and Archaeology Curator Don Wyckoff Old Field Jacket Yields New Donors to Retire Species Wyckoff Receives Citation of Merit DONATION …………………………… 3 EDUCATION ……………………… 10 Redbud Café Welcomes New Whitten-Newman Foundation Funds Education Grants Fund Programs Chef Exhibit Former Curator Sidney DeVere UPCOMING EVENTS ………… 11 Brown Dies EXHIBIT ……………………………… 4-5 Art and the Animal Museum Welcomes 2011 Black Mesa Exhibit Open Summer Explorers Corporate Partners Baby Apatosaurus Longtime Volunteers Pass On COLLECTIONS ………………… 6-7 Bill Miller Named Volunteer of New ‘Thunder-Thighs’ Dinosaur the Year Discovered TRACKS, SPRING 2011 : VOLUME 23 NO. 1 1 FROM THE DIRECTOR Dear Members and Friends, On March 4 we had a very special ribbon cutting reception for our newest permanent exhibit in the museum—Black Mesa. We started this project in the 1990s, but the construction of this exhibit was made possible by a $1 million gift from Reggie and Rachelle Whitten and the Whitten-Newman Foundation. Located in the Hall of Natural Wonders, Black Mesa is a 1,500 square foot diorama of Oklahoma’s Panhandle, home to the highest elevation in the state. When you walk into the Hall of Natural Wonders, you can now view major habitats of our state from the forest to the prairie to the Black Mesa. -

Comissão Organizadora
ISSN 2175-7720 Paleontologia 2 Livro de Resumos ISSN 2175-7720 Paleontologia 3 Livro de Resumos Comitê Editorial: Valéria Gallo Hilda Maria Andrade da Silva Capa e Identidade Visual: Mapinguari Design Projeto gráfico interno e editoração: Rafael Fernandes Lopes da Silva XXI Congresso Brasileiro de Paleontologia: A paleontologia e os eventos globais. 2009, Belém, Pará, Brasil. ISSN: 2175-7720 1. Paleontologia 2. Geociências 3. Congresso Brasileiro de Paleontologia Paleontologia 4 Sobre a Logomarca A Logomarca do XXI Congresso Brasileiro de Paleontologia O gênero Orthaulax é um representante extinto da família Strombidae, endêmico da província paleobiogeográfica Caribeana, vivente entre o Oligoceno Superior e o Mioceno Inferior. Do ponto de vista paleoambiental teria vivido em ambiente marinho de águas rasas, quentes, límpidas, agitadas e com salinidade normal. O processo envolvente da sua ultima volta tornou a concha bastante sólida e maciça, permitindo que habitasse ambiente de grande agitação, como os biohermas, pequenas edificações recifais. Na Formação Pirabas, a espécie Orthaulax pugnax (Heilprin, 1887) foi reconhecida por Maury (1925), e corroborada em pesquisas subseqüentes. Estudos realizados por Cândido Simões Ferreira, delimitaram nos calcários aflorantes no litoral nordeste do Estado do Pará e noroeste do Estado do Maranhão, uma zona caracterizada por elementos estenobiônticos, típicos de recifes de corais. A espécie O. pugnax, associada com algas coralíneas, corais hermatípicos e equinóides regulares são as formas mais características que contribuíram para a edificação do bioherma. Assim, este gastrópode constitui-se em um elemento importante da Formação Pirabas, por ter sido o primeiro táxon utilizado para datar esta unidade litoestratigráfica como oligo-miocênica, bem como seu decisivo papel para correlação com outras unidades sincrônicas da Província Biogeográfica Caribeana, permitindo delimitar no norte do Brasil, a sua extremidade sul. -

Dinosaur Discovered (W/ Video) 23 February 2011
New 'thunder-thighs' dinosaur discovered (w/ Video) 23 February 2011 speculate that the larger specimen is the mother of the younger and would have weighed around 6 tons, about the size of a large elephant, and measured 14 meters in length. At a third of the size, the smaller specimen would have weighed about 200 kg, the size of a pony, and been 4.5 m long. The authors classified the new genus based on an incomplete skeleton including bones from the shoulder, hip, ribs, vertebrae and some unidentifiable fragments. They used the bones to identify Brontomerus' unique features, primarily the Brontomerus mcintoshi is a newly discovered dinosaur shape of the ilium (hip bone), which, in the case of from the Early Cretaceous of North America. The name Brontomerus means "Thunder thighs" -- a name chosen Brontomerus, is unusually large in comparison to because the peculiar shape of the hip bone shows that it that of similar dinosaurs. The wide, blade-shaped would have had enormously powerful thigh muscles in bone projects forward ahead of the hip socket, life. providing a proportionally massive area for the attachment of muscles. The shape of the bone indicates that the animal (PhysOrg.com) -- A new dinosaur named would likely have had the largest leg muscles of Brontomerus mcintoshi, or "thunder-thighs" after its any dinosaur in the sauropod family. This is enormously powerful thigh muscles, has been reflected in the name Brontomerus, which literally discovered in Utah, USA. The new species is means "thunder-thighs." The dinosaur's species described in a paper recently published in the name, mcintoshi, was chosen in honor of John journal Acta Palaeontologica Polonica by an "Jack" McIntosh, a retired physicist at Wesleyan international team of scientists from the U.K. -

Titanosauriform Teeth from the Cretaceous of Japan
“main” — 2011/2/10 — 15:59 — page 247 — #1 Anais da Academia Brasileira de Ciências (2011) 83(1): 247-265 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 www.scielo.br/aabc Titanosauriform teeth from the Cretaceous of Japan HARUO SAEGUSA1 and YUKIMITSU TOMIDA2 1Museum of Nature and Human Activities, Hyogo, Yayoigaoka 6, Sanda, 669-1546, Japan 2National Museum of Nature and Science, 3-23-1 Hyakunin-cho, Shinjuku-ku, Tokyo 169-0073, Japan Manuscript received on October 25, 2010; accepted for publication on January 7, 2011 ABSTRACT Sauropod teeth from six localities in Japan were reexamined. Basal titanosauriforms were present in Japan during the Early Cretaceous before Aptian, and there is the possibility that the Brachiosauridae may have been included. Basal titanosauriforms with peg-like teeth were present during the “mid” Cretaceous, while the Titanosauria with peg-like teeth was present during the middle of Late Cretaceous. Recent excavations of Cretaceous sauropods in Asia showed that multiple lineages of sauropods lived throughout the Cretaceous in Asia. Japanese fossil records of sauropods are conformable with this hypothesis. Key words: Sauropod, Titanosauriforms, tooth, Cretaceous, Japan. INTRODUCTION humerus from the Upper Cretaceous Miyako Group at Moshi, Iwaizumi Town, Iwate Pref. (Hasegawa et al. Although more than twenty four dinosaur fossil local- 1991), all other localities provided fossil teeth (Tomida ities have been known in Japan (Azuma and Tomida et al. 2001, Tomida and Tsumura 2006, Saegusa et al. 1998, Kobayashi et al. 2006, Saegusa et al. 2008, Ohara 2008, Azuma and Shibata 2010).