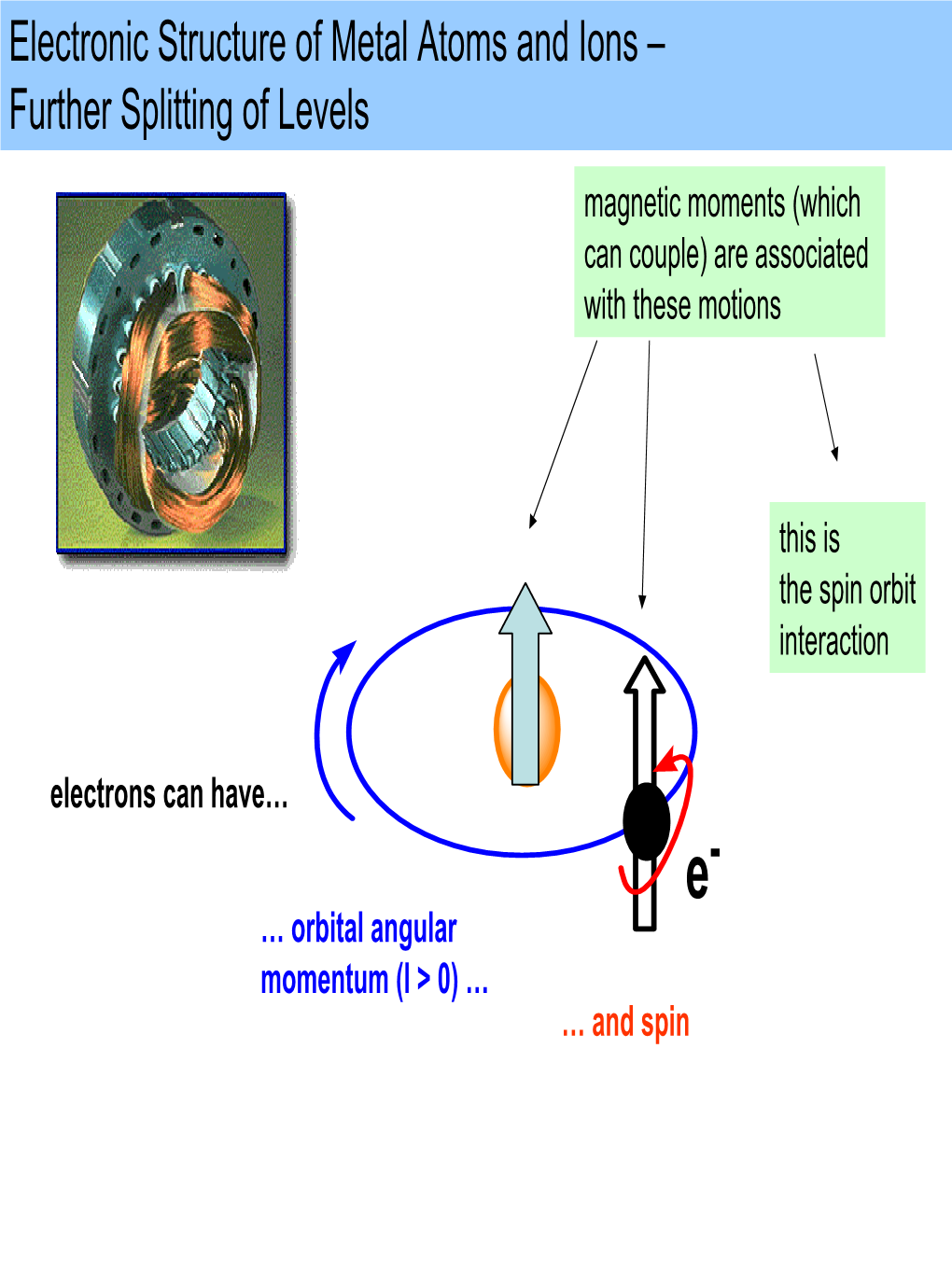
(Which Can Couple) Are Associated with These Motions This Is the Spin Orbit Interaction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Quantum Spectropolarimetry and the Sun's Hidden
QUANTUM SPECTROPOLARIMETRY AND THE SUN’S HIDDEN MAGNETISM Javier Trujillo Bueno∗ Instituto de Astrof´ısica de Canarias; 38205 La Laguna; Tenerife; Spain ABSTRACT The dynamic jets that we call spicules. • The magnetic fields that confine the plasma of solar • Solar physicists can now proclaim with confidence that prominences. with the Zeeman effect and the available telescopes we The magnetism of the solar transition region and can see only 1% of the complex magnetism of the Sun. • corona. This is indeed∼regrettable because many of the key prob- lems of solar and stellar physics, such as the magnetic coupling to the outer atmosphere and the coronal heat- Unfortunately, our empirical knowledge of the Sun’s hid- ing, will only be solved after deciphering how signifi- den magnetism is still very primitive, especially concern- cant is the small-scale magnetic activity of the remain- ing the outer solar atmosphere (chromosphere, transition ing 99%. The first part of this paper1 presents a gentle ∼ region and corona). This is very regrettable because many introduction to Quantum Spectropolarimetry, emphasiz- of the physical challenges of solar and stellar physics ing the importance of developing reliable diagnostic tools arise precisely from magnetic processes taking place in that take proper account of the Paschen-Back effect, scat- such outer regions. tering polarization and the Hanle effect. The second part of the article highlights how the application of quantum One option to improve the situation is to work hard to spectropolarimetry to solar physics has the potential to achieve a powerful (European?) ground-based solar fa- revolutionize our empirical understanding of the Sun’s cility optimized for high-resolution spectropolarimetric hidden magnetism. -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

The Zeeman Effect
THE ZEEMAN EFFECT OBJECT: To measure how an applied magnetic field affects the optical emission spectra of mercury vapor and neon. The results are compared with the expectations derived from the vector model for the addition of atomic angular momenta. A value of the electron charge to mass ratio, e/m, is derived from the data. Background: Electrons in atoms can be characterized by a unique set of discrete energy states. When excited through heating or electron bombardment in a discharge tube, the atom is excited to a level above the ground state. When returning to a lower energy state, it emits the extra energy as a photon whose energy corresponds to the difference in energy between the two states. The emitted light forms a discrete spectrum, reflecting the quantized nature of the energy levels. In the presence of a magnetic field, these energy levels can shift. This effect is known as the Zeeman effect. Zeeman discovered the effect in 1896 and obtained the charge to mass ratio e/m of the electron (one year before Thompson’s measurement) by measuring the spectral line broadening of a sodium discharge tube in a magnetic field. At the time neither the existence of the electron nor nucleus were known. Quantum mechanics was not to be invented for another decade. Zeeman’s colleague, Lorentz, was able to explain the observation by postulating the existence of a moving “corpuscular charge” that radiates electromagnetic waves. (See supplementary reading on the discovery) Qualitatively the Zeeman effect can be understood as follows. In an atomic energy state, an electron orbits around the nucleus of the atom and has a magnetic dipole moment associated with its angular momentum. -

Landau Quantization, Rashba Spin-Orbit Coupling and Zeeman Splitting of Two-Dimensional Heavy Holes
Landau quantization, Rashba spin-orbit coupling and Zeeman splitting of two-dimensional heavy holes S.A.Moskalenko1, I.V.Podlesny1, E.V.Dumanov1, M.A.Liberman2,3, B.V.Novikov4 1Institute of Applied Physics of the Academy of Sciences of Moldova, Academic Str. 5, Chisinau 2028, Republic of Moldova 2Nordita, KTH Royal Institute of Technology and Stockholm University, Roslagstullsbacken 23, 10691 Stockholm, Sweden 3Moscow Institute of Physics and Technology, Inststitutskii per. 9, Dolgoprudnyi, Moskovsk. obl.,141700, Russia 4Department of Solid State Physics, Institute of Physics, St.Petersburg State University, 1, Ulyanovskaya str., Petrodvorets, 198504 St. Petersburg, Russia Abstract The origin of the g-factor of the two-dimensional (2D) electrons and holes moving in the periodic crystal lattice potential with the perpendicular magnetic and electric fields is discussed. The Pauli equation describing the Landau quantization accompanied by the Rashba spin-orbit coupling (RSOC) and Zeeman splitting (ZS) for 2D heavy holes with nonparabolic dispersion law is solved exactly. The solutions have the form of the pairs of the Landau quantization levels due to the spinor-type wave functions. The energy levels depend on amplitudes of the magnetic and electric fields, on the g-factor gh , and on the parameter of nonparabolicity C . The dependences of two mh energy levels in any pair on the Zeeman parameter Zghh , where mh is the hole effective 4m0 mass, are nonmonotonous and without intersections. The smallest distance between them at C 0 takes place at the value Zh n /2, where n is the order of the chirality terms determined by the RSOC and is the same for any quantum number of the Landau quantization. -

Sun – Part 20 – Solar Magnetic Field 2 – Zeeman Effect
Sun – Part 20 - Solar magnetic field 2 – Zeeman effect The Zeeman effect The effect in a solar spectral line Pieter Zeeman This is a means of measuring a magnetic field from a distance, one that is particularly useful in astronomy where such measurements for stars have to be made indirectly. Use of the Zeeman effect is particularly important in relation to the Sun's magnetic field because of the central role played by the solar magnetic field in solar activity eg solar flares, coronal mass ejections, which can, potentially, pose a serious hazard to many aspects of human life. The Zeeman effect is the splitting of a spectral line into several components due to the presence of a static magnetic field. Normally, atoms in a star’s atmosphere will absorb a certain frequency of energy in the electromagnetic spectrum. However, when the atoms are within a magnetic field, these lines become split into multiple, closely spaced lines. For example, under normal conditions, an atomic spectral line of 400nm would be single. However, in a strong magnetic field, because of the Zeeman effect, the line would be split to yield a more energetic line and a less energetic line, in addition to the original line. The distance between the splits is a function of the magnetic field, so this effect can be used to measure the magnetic fields of the Sun and other stars. The energy also becomes polarised, with an orientation depending on the orientation of the magnetic field. Thus, strength and direction of a star’s magnetic field can be determined by study of Zeeman effect lines. -

The Zeeman Effect
The Zeeman Effect Many elements of this lab are taken from “Experiments in Modern Physics” by A. Melissinos. Intro ! The mercury lamp may emit ultraviolet radiation that is damaging to the cornea: Do NOT look directly at the lamp while it is lit. Always view the lamp through a piece of ordinary glass or through the optics chain of the experiment. Ordinary glass absorbs ultraviolet radiation. The mercury lamp’s housing however may be quartz glass which doesn’t absorb the ultraviolet. ! Don’t force anything. ! If you are unsure, ask first. In this lab, you will measure the Bohr Magneton by observing the Zeeman splitting of the mercury emission line at 546.1 nm. To observe this tiny effect, you will apply a high-resolution spectroscopy technique utilizing a Fabri-Perot cavity as the dispersive element. The most difficult aspect of this experiment is the interpretation of the Fabri-Perot cavity output and understanding the underlying theory. It’s easy to confuse the many different “effects” modifying the energy of electrons orbiting the nucleous (and therefore affecting atomic spectra) so here is a short guide to the various types: 1 o Basic level structure – This is the familiar structure most of us learn first in chemistry class whereby electrons arrange themselves in orbital “shells” around the nucleus. The orbitals have a number assigned to them, corresponding very roughly to their distance from the nucleus: 1, 2, 3 etc. They also have a letter assigned to them corresponding to the shape of the orbital: S, P, D, .. etc (S shells are spherical, P shells look like two teardrops with the pointy ends touching, etc.) Each S shell can hold 2 electrons, each P shell can hold 6, each D shell 10 and so on. -

The Zeeman Effect
The Zeeman Effect Robert DeSerio Ph 461 Objective: In this experiment, you will measure the small energy shifts in the magnetic sublevels of atoms in "weak" magnetic fields. The visible light from transitions between various multiplets is dispersed in a 1.5 m grating spectrograph and observed with a telescope. Each observed line consists of a group of closely spaced unresolved lines from the emissions among the different magnetic sublevels within each multiplet. Using a polarizer and Fabry-Perot interferometer, ring patterns are observed within each spectro graphically resolved line. The patterns are measured as a function of the strength of the magnetic field to determine the g-factor of the multiplet. 1 Theory 1.1 Magnetic moment The electron (charge -e, mass m) has an intrinsic spin angular momentum 8 and an intrinsic magnetic moment Its that can be expressed as: flB Its = -2--';8 (1) where flB = eli/2mc is called the Bohr magneton and is effectively the atomic unit of magnetic moment. (The factor 2 in Eqn. 1 is a prediction of Dirac's relativistic theory of electron spin, and when quantum electrodynamic effects are included increases by about 0.1 %) If a spinless electron is orbiting a nucleus in a state of angular momentum L, it behaves like a current loop with a magnetic moment It.e proportional to 1 2 Modern Physics Laboratory i: J1B J.t.e = --i, (2) 'Ii The nucleus also often has a magnetic moment, but because of the large nuclear mass, it is much smaller than that of the electron, and at the resolu tions involved in this experiment its effects will be unobservable. -

Lecture 22 Relativistic Quantum Mechanics Background
Lecture 22 Relativistic Quantum Mechanics Background Why study relativistic quantum mechanics? 1 Many experimental phenomena cannot be understood within purely non-relativistic domain. e.g. quantum mechanical spin, emergence of new sub-atomic particles, etc. 2 New phenomena appear at relativistic velocities. e.g. particle production, antiparticles, etc. 3 Aesthetically and intellectually it would be profoundly unsatisfactory if relativity and quantum mechanics could not be united. Background When is a particle relativistic? 1 When velocity approaches speed of light c or, more intrinsically, when energy is large compared to rest mass energy, mc2. e.g. protons at CERN are accelerated to energies of ca. 300GeV (1GeV= 109eV) much larger than rest mass energy, 0.94 GeV. 2 Photons have zero rest mass and always travel at the speed of light – they are never non-relativistic! Background What new phenomena occur? 1 Particle production e.g. electron-positron pairs by energetic γ-rays in matter. 2 Vacuum instability: If binding energy of electron 2 4 Z e m 2 Ebind = > 2mc 2!2 a nucleus with initially no electrons is instantly screened by creation of electron/positron pairs from vacuum 3 Spin: emerges naturally from relativistic formulation Background When does relativity intrude on QM? 1 When E mc2, i.e. p mc kin ∼ ∼ 2 From uncertainty relation, ∆x∆p > h, this translates to a length h ∆x > = λ mc c the Compton wavelength. 3 for massless particles, λc = , i.e. relativity always important for, e.g., photons. ∞ Relativistic quantum mechanics: outline -

Zeeman Effect in Na, Cd, and Hg Masatsugu Sei Suzuki and Itsuko S
Lecture Note on Senior Laboratory Zeeman effect in Na, Cd, and Hg Masatsugu Sei Suzuki and Itsuko S. Suzuki Department of Physics, SUNY at Binghamton (Date March 13, 2011) Abstract: In 1897, Pieter Zeeman observed the splitting of the atomic spectrum of cadmium (Cd) from one main line to three lines. Such a splitting of lines is called the normal Zeeman effect. According to the oscillation model by Hendrik Lorentz, the Zeeman splitting arises from the oscillation of charged particles in atoms. The discovery of the Zeeman effect indicates that the charged particles are electrons. In 1897 - 1899, J.J. (Joseph John) Thomson independently found the existence of electrons from his explorations on the properties of cathode rays. There are few atoms showing the normal Zeeman effect. In contrast, many atoms shows anomalous Zeeman effects. For the spectra of sodium (Na). for example, there are two D- lines (yellow) in the absence of the magnetic field. When the magnetic field is applied, each line is split into four and six lines, respectively. Although Zeeman himself observed the spectra of Na, he could not find the splitting of the D lines in the presence of the magnetic field because of the low resolution of his spectrometer. The electron configuration of Na is similar to that of hydrogen. There is one electron outside the closed shell. Instead, he chose Cd for his experiment and found the normal Zeeman effect (three lines). In the electron configuration of Cd, there are two electrons outside the closed shell. The three lines observed in Cd was successfully explained in terms of the Lorentz theory. -

Zeeman Effect
Zeeman effect F44 Max-Planck-Institut für Kernphysik Saupfercheckweg 1, Heidelberg Introduction The most important experimental technique triggering the development of the modern atomic theory is spectroscopy, the observation of the characteristic wavelengths of light that atoms can absorb and emit. A so-called wavelength dispersive element such as a water drop, a prism or a grating, spatially separates different wavelengths of light emitted from one source. If the light source emits white light, a band of all colours will be found after the light passes the wavelength dispersive element (e.g. rainbow). Observing the light from a lamp that contains only atoms of a certain element, one finds distinct lines instead of the complete band on the detector. The positioning of the lines on the detector is called a spectrum, and the corresponding wavelengths are hence called "spectral lines"of the element. The additional information needed to be able to draw conclusions about the atomic structure from spectral lines was provided by the experimental observation of Rutherford that in an atom, electrons surround a heavy, positively charged nucleus in the center, and by Planck’s explanation of the blackbody spectrum by quanta of light, photons, whose energy Eph is connected with the wavelength l by hc E = hn = (1) ph l where n is the frequency, h is the Planck constant and c is the speed of light. With this knowledge it became clear that electrons in an atom can occupy only certain energy levels Ei, and that the transition of an electron from a higher energy level E2 to a lower energy level E1 leads to the emission of a photon: E2 − E1 = Eph. -

The Zeeman-Effect
SomeSome BasicsBasics QuantumQuantum numbersnumbers (qn)(qn) nn = 1, 2, 3, ¼ main qn (energy states -shell) ll = 0, 1, ¼ (n-1) orbital angular momentum (s, p, d, ¼-subshell) mml = -l, ¼, +l magnetic qn, projection of ll (energy shift) mms = ±½ spin angular momentun jj = |l-sl-s | ≤ jj ≤ l+sl+s total angular momentum (if coupled, interactions of spin-orbit dominate over B) SomeSome BasicsBasics • l = 0, ml = 0 • l = 1, ml = 0, 1 » l = 2, ml = 0, 1, 2 SomeSome BasicsBasics moremore thanthan 11 ee - Eigenvalues:Eigenvalues: L= Σ l (S,(S, P,P, D,D, ¼)¼) ½ L= Σ li LL== ħħ [[ll ((ll +1)]+1)]½ S=S= ΣΣ ssi SS== ħħ [[ss ((ss +1)]+1)]½ ½ J=J= ΣΣ jji (with(with mmJ)) JJ== ħħ [[jj ((jj +1)]+1)] ZeemanZeeman EffectEffect • discovered 1896 different splitting patterns in different spectral lines • splitted lines are polarized • splitting proportional to magnetic field 2 Δλ ~ gL λ B • Interaction of atoms with external magnetic field due to magnetic moment mj ZeemanZeeman EffectEffect • normal Zeeman effect: S = 0, L = J ≠ 0 • split in 3 components components with ΔmJ = 0 (π ) components with ΔmJ = -1 and +1 (± σ) • 2 possibilities: • B parallel to line of sight two σ circular polarized • B perpendicular to line of sight two σ polarised vertical to field one π polarised horizontal to field ZeemanZeeman EffectEffect interaction with external field – magnetic momentum of Atom • J and μJ antiparallel since S=0 and L=J gJ = 1 only 3 values of Δm possible: 0, ± 1 π, ± σ μB = (eħ)/(2me) Bohr magneton V = mJμBB0 ZeemanZeeman EffectEffect Selection