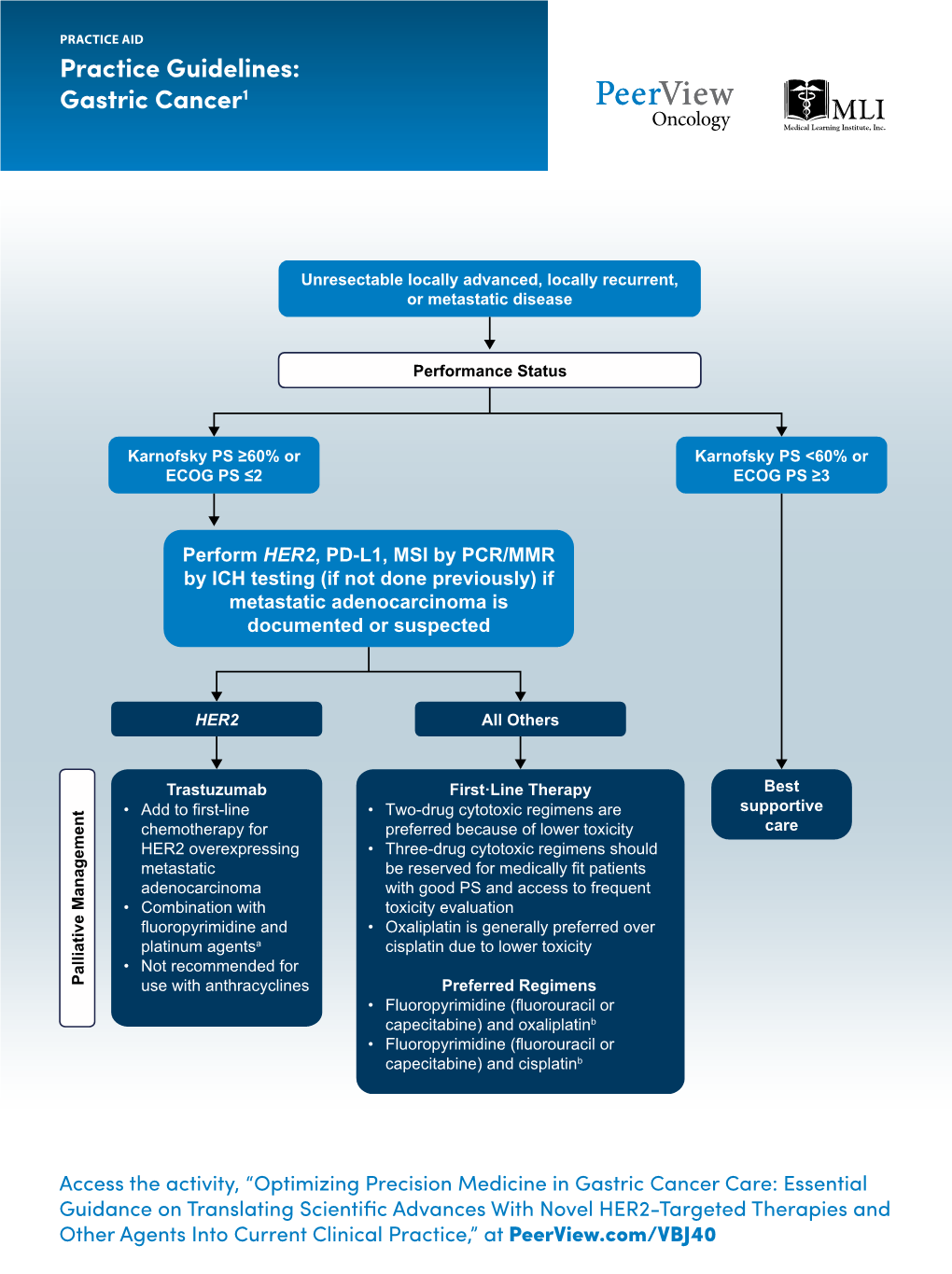
Practice Guidelines: Gastric Cancer1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

The Pipeline Report 2016 Pipeline 2014 Autoimmune
THE PIPELINE REPORT 2016 PIPELINE 2014 AUTOIMMUNE PRODUCTS GENERATING BUZZ OTHER KEY PRODUCTS IN THE PIPELINE BIG-TIME Baricitinib Eli Lilly/Incyte Indication: RA (Ph.III) Romosozumab Amgen/UCB Sirukumab Janssen Biotech RA What the clinical trials found: The daily oral demonstrated superiority Osteoporosis (Ph.III) (Ph.III) compared to placebo after 12 weeks based on ACR20 response (Ph. Avatrombopag Astellas Pharma Anifrolumab Medarex/Med III RA-BEAM). The agent also proved superior to adalimumab on ITP/thrombocytopenia (Ph.III) Immune Systemic lupus erythema- tosus (Ph.III) key secondary objectives of ACR20 response and improvement in Elobixibat AstraZeneca CIC and DAS28-hsCRP score. A few occasional AEs were reported. IBS-C (Ph.III) Odanacatib Merck Osteoporosis (Ph.III) Credit Suisse Success Probability and inThought Comment: 70%. Lesinurad AstraZeneca Gout (Ph. III) Tildrakizumab Merck Psoriasis The JAK inhibitor appears to have similar efficacy and safety to (Ph.III) Pfizer’s Xeljanz. It was supposed to have a once daily vs. Xeljanz’s Alicaforsen Atlantic Healthcare Pouchitis/ulcerative colitis (Ph.III) Siponimod Novartis MS (Ph.III) twice daily advantage, but Xeljanz’s once daily formulation will likely be approved soon. It’ll be interesting to see if Lilly/Incyte Rituximab biosimilar Boehringer Infliximab biosimilar Pfizer RA Ingelheim RA (Ph.III) (Ph.III) can do something with patient access and price to improve upon Mongersen Celgene/Nogra RHB 104 RedHill Biopharma the poor performance of Xeljanz and expand the JAK inhibitor Pharma Crohn’s disease (Ph.III) Crohn’s disease (Ph.III) market. Expected launch: 2016 (Source: Credit Suisse) Etanercept biosimilar Coherus Sarilumad Regeneron RA (Ph.III) Credit Suisse forecast: $1.09 billion in global annual sales by 2020 Biosciences/Daiichi Sankyo/ Etrolizumab Roche Ulcerative A peek at 159 aspiring agents, with profiles on 17 that could shoot to stardom. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Transformatıon
ANNUAL REPORT 2012 a time of Transformatıon 238038_Peregrine_Cov.indd 3 8/23/12 8:38 PM Transforming Our Business · Two Phase III-Ready Drug Candidates: Bavituximab and Cotara® · Compelling Bavituximab Proof-of-Concept Clinical Data in Non-Small Cell Lung Cancer · Broad Bavituximab Oncology Program with Multiple Indications Under Clinical Evaluation · Integrated and Growing Commercial Manufacturing Business Transforming Patients’ Lives · Treating Diseases with High Unmet Medical Needs Including NSCLC and GBM · Comprehensive Development Strategy Exploring Broad Potential of Bavituximab in Oncology · Developing New Diagnostics with Potential Applications in Many Life-threatening Diseases · Helping Bring Important New Treatments and Diagnostics to the Market through our Manufacturing Capabilities and Expertise Peregrine’s Advancing Oncology Pipeline PRECLINICAL PHASE I PHASE II PHASE III Bavituximab NSCLC Second-Line NSCLC Front-Line Pancreatic Front-Line Advanced Liver Cancer (HCC) IST Prostate Cancer (CRPC) IST NSCLC Front-Line IST Breast (HER!-Negative) IST Rectal Adenocarcinoma IST PGN!"# PS-Targeted Tumor Imaging Cotara® Recurrent GBM Transforming Our Future With two Phase III-ready programs, an expanding pipeline and a successful commercial manufacturing business, Peregrine is transitioning from an early-stage to a late-stage development company. The company is in a unique position of strength on several fronts with multiple partnering, development and revenue-generating opportunities. These opportunities can help not only transform the company, but potentially the lives of patients with life-threatening diseases. Dear Stockholders, This is a truly transformative time for Peregrine. We are transitioning from an early-stage research organization to a late-stage drug development company, thanks to an expanding product pipeline that now includes two Phase III-ready programs, exceptional proof-of-concept clinical data for our lead product candidate bavituximab and a record-breaking revenue year for our contract manufacturing business. -

Targeting Angiogenesis in Advanced Non-Small Cell Lung Cancer
Original 1235 Article CE Targeting Angiogenesis in Advanced Non–Small Cell Lung Cancer Philip E. Lammers, MD, MSCI, and Leora Horn, MD, MSc Abstract stage IIIB/IV adenocarcinoma have a molecular muta- Lung cancer is the leading cause of cancer-related mortality in the tion thought to drive tumor growth.4 However, only United States. Over the past 40 years, treatments with standard patients with epidermal growth factor receptor (EGFR) chemotherapy agents have not resulted in substantial improve- ments in long-term survival for patients with advanced lung can- mutations (approximately 10%–15%) or anaplastic lym- cer. Therefore, new targets have been sought, and angiogenesis is phoma kinase (ALK) rearrangements (approximately a promising target for non–small cell lung cancer (NSCLC). Bevaci- 5%) have an FDA-approved therapy available. Other zumab, a monoclonal antibody targeted against the vascular en- potential targets have been identified, such as c-ros on- dothelial growth factor, is the only antiangiogenic agent currently cogene 1 (ROS1) gene fusions and BRAF mutations, recommended by NCCN for the treatment of advanced NSCLC. However, several antibody-based therapies and multitargeted ty- and clinical trials using targeted agents are ongoing. rosine kinase inhibitors are currently under investigation for the Alternative targets continue to be investigated, one of treatment of patients with NSCLC. This article summarizes the avail- which is angiogenesis, a necessary process in the growth able clinical trial data on the efficacy and safety of these agents in and metastasis of solid tumors.5 Bevacizumab is the only patients with advanced lung cancer. (JNCCN 2013;11:1235–1247) antiangiogenic therapy FDA-approved for NSCLC,6 but other agents have been tested in advanced NSCLC. -

Ep 3178848 A1
(19) TZZ¥__T (11) EP 3 178 848 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 14.06.2017 Bulletin 2017/24 C07K 16/28 (2006.01) A61K 39/395 (2006.01) C07K 16/30 (2006.01) (21) Application number: 15198715.3 (22) Date of filing: 09.12.2015 (84) Designated Contracting States: (72) Inventor: The designation of the inventor has not AL AT BE BG CH CY CZ DE DK EE ES FI FR GB yet been filed GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (74) Representative: Cueni, Leah Noëmi et al Designated Extension States: F. Hoffmann-La Roche AG BA ME Patent Department Designated Validation States: Grenzacherstrasse 124 MA MD 4070 Basel (CH) (71) Applicant: F. Hoffmann-La Roche AG 4070 Basel (CH) (54) TYPE II ANTI-CD20 ANTIBODY FOR REDUCING FORMATION OF ANTI-DRUG ANTIBODIES (57) The present invention relates to methods of treating a disease, and methods for reduction of the formation of anti-drug antibodies (ADAs) in response to the administration of a therapeutic agent comprising administration of a Type II anti-CD20 antibody, e.g. obinutuzumab, to the subject prior to administration of the therapeutic agent. EP 3 178 848 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 178 848 A1 Description Field of the Invention 5 [0001] The present invention relates to methods of treating a disease, and methods for reduction of the formation of anti-drug antibodies (ADAs) in response to the administration of a therapeutic agent. -

OHC Clinical Trials List V4
FOR MORE INFORMATION OR TO REFER A PATIENT TO ANY OF OUR CLINICAL TRIALS, PLEASE CONTACT NICOLE GIVEN, BS, OHC RESEARCH DEPARTMENT AT 1-800-710-4674 Phase 1 SOLID TUMOR BRE-261: GO29831 (BAM Only) A Phase Ib, Randomized, Two-Arm Study Evaluating the Safety and Phase Ib Pharmacokinetics of MPDL328A (Anti-PD-L1 Antibody) in Combination with Therapeutic Trastuzumab Emtansine or Trastuzumab and Pertuzumab in Patients with MPDL3280A provided HER2-Positive Breast Cancer GI-219: BBI608-246 (pending 1st Qtr) A Phase Ib Clinical Study of BBI608 in Combination with Standard Phase Ib Chemotherapies in Adult Patients with Advanced Gastrointestinal Cancer Therapeutic BBI608 provided REFMAL-381: ABI-007-ST-001 (BAM Only) Phase 1, Open-Label, Multi-Center, Safety Study of Nivolumab (BMS- Phase I 936558) in Combination with Nab-Paclitaxel Plus or Minus Gemcitabine in Therapeutic Pancreatic Cancer, Nab-Paclitaxel/Carboplatin in Stage IIIB/IV Non-Small Nivolumab, Abraxane provided Cell Lung Cancer or Nab-Paclitaxel in Recurrent Metastatic Breast Cancer REFMAL-404: TGR-1202-102 (BAM Only) A Phase I Study Evaluating the Safety and Efficacy of TGR 1202 Alone and in Phase I Combination with Either Nab-Paclitaxel + Gemcitabine or with FOLFOX in Therapeutic Patients with Select Relapsed or Refractory Solid Tumors TGR-1202 provided HEMATOLOGY MM-56: CFZ013 Phase Ib Study of Carfilzomib Administered Once Weekly in Combination Phase Ib with Lenalidomide and Dexamethasone in Subjects with Multiple Myeloma Therapeutic Carfilzomib provided Page 1 of 20 January 11, -

(INN) for Biological and Biotechnological Substances
INN Working Document 05.179 Update 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

KOL Event & Corporate Update
KOL Event & Corporate Update April 6, 2017 NASDAQ: VBLT Agenda Opening remarks Dr. Ramy Ibrahim, MD – Parker institute for Cancer Immunotherapy New Targets and Immuno‐therapeutic Approaches in Oncology Prof. Dror Harats, MD – CEO, VBL Therapeutics VBL’s Novel Approach to Immune‐Oncology 2 Use of checkpoint modulators in Advanced malignancies Ramy Ibrahim, MD Head of Clinical Development Disclosures Paid consultant for Arcus therapeutics Honorarium from PER History and evolution of immunotherapy Immunotherapy Delivers In 2013, Science selected cancer immunotherapy as its Breakthrough of the Year. In 2014, the Wall Street Journal showcased cancer patients who were treated with immunotherapies and survived years beyond their initial prognosis. Ambitious goal with immunotherapy Traditional Therapies Immunotherapy 100% 100% Long Term Benefit Combinations of Traditional Immunotherapy Cancer Therapies Alive Alive Immunotherapy % of Treated %Patients of Treated % of Treated %Patients of Treated Traditional Therapies 0% 0% Time Time Patients ultimately succumb to their disease 2011: Immunotherapy delivered Long term benefit: Only 1in every 5 treated patients Mechanism of Action is simple… MEMORY cell Cancer cell It is more complicated…many pathways Some activate , others inhibit but many remain to be unknown Unleashing Immunotherapy’s Potential Multi disciplinary Genomics Immunomics Proteomics Microbiome Clinical Outcomes Data Science >1,250+ companies Biology >3,000 IO trials Oncology 100’s of research institutions Physics + Chemistry 100’s of -

OHC Clinical Trials List 11-14
FOR MORE INFORMATION OR TO REFER A PATIENT TO ANY OF OUR CLINICAL TRIALS, CONTACT NICOLE GIVEN, BS, OHC RESEARCH DEPARTMENT AT 800-710-4674 ext. 27110 Molecular Profiling PRO-02: ML28897 MY PATHWAY: An OPen-Label Phase IIA Study Evaluating Trastuzumab/ Phase IIA Pertuzumab, Erlotinib, Vemurafenib, and Vismodegib in Patients who Have Therapeutic Advanced Solid Tumors with Mutations or Gene Expression Abnormalities Trastuzumab, Pertuzumab, Erlotinib, Predictive of ResPonse to One of These Agents Vemurafenib, Vismodegib Provided PRO-03: CBKM120ZUS40 (pending) Modular Phase II studY to link targeted theraPY to Patients with PathwaY Phase II activated tumors: Module 1 – BKM120 for patients with PI3K-activated Therapeutic tumors BKM120 provided PRO-04: CTKI258AUS26 Modular Phase II studY to link targeted theraPY to Patients with PathwaY Phase II activated tumors: Module 2 – Dovitinib for Patients with tumor PathwaY Therapeutic activations inhibited bY dovitinib including tumors with mutations or Dovitinib Provided translocations of FGFR, PDGFR, VEGF, cKIT, FLT3, CSFR1, Trk and RET PRO-06: CMEK162AUS11 (Pending) Modular Phase II studY to link targeted theraPY to patients with pathwaY Phase II activated tumors: Module 3 – MEK162 for Patients with RAS/RAF/MEK Therapeutic activated tumors MEK162 Provided PRO-07: CLGX818AUS03 (Pending) Modular Phase II studY to link targeted theraPY to Patients with PathwaY Phase II activated tumors: Module 4 – LGX818 for Patients with BRAFV600 mutated Therapeutic tumors LGX818 Provided PRO-08: CLDE225XUS20 (Pending)