Anti-Platelet Effect of Bryophyllum Pinnatum Aqueous Extract in Human Blood
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

11Th and Final Report 7-6-2017.Xlsx
List of Young Scientists (under 40 years of age) Directory of the Productive Scientists of Pakistan 2016 Name/ Designation/ Department/ S.No Sci ID -index ARO Total Organization Score Score Score Score Score Score Score Score Score Score Score (ARO) Books Edited Factor h Published/ Total PhDs Applicant's Applicant's Res. Grants Publications Total Impact 5% 5% 0f Scaled 5% 0f Scaled 5% 0f Scaled 5% 0f Scaled Total Applied Total Patents Total Awards Total Awards Patents score Impact Factor 10% 0f Scaled Total External 30% 0f Scaled 15% 0f Scaled 15% 0f Scaled 10% 0f Scaled Grand Score Total Citations Total Score for Total Score for Total Score for Citations Score Score for Books PhD Supervision Research Grants Research Output F. Health Sciences Shoaib Ahmad Malik (Dr.) Assistant Professor 1 2875 0 0 0.000 0 0 0.00 0 0 0.00 0 0 21 172.3 14.848 0.47 1253 118.001 0.34 15 3.63 0 0 0.00 0 0 0.00 Sargodha Medical College, 0.00 4.44 University of Sargodha, Sargodha Muhammad Arif Lodhi (Dr.) Assistant Professor 2 170 0 0 0.000 0 0 0.00 0 0 0.00 3 7.5 53 63.007 8.792 0.28 514 65.71 0.19 12 2.90 0 0 0.00 0 0 0.00 Department of Biochemistry, 0.07 3.44 Abdul Wali Khan University, Mardan Shah Jahan (Dr.) Assistant Professor 3 119 0 0 0.000 0 0 0.00 0 0 0.00 2 5 22 54.965 9.633 0.31 237 40.1059 0.12 10 2.42 0 0 0.00 0 0 0.00 Department of Immunology, 0.05 2.90 University of Health Sciences, Lahore Sobia Manzoor (Dr.) Assistant Professor 4 Department of Healthcare Biotechnology, 28561 0 0 0.000 0 0 0.00 2 10 0.12 0 0 0.00 28 52.556 8.329 0.26 172 24.808 -

Prevalence and Severity of Depressive Illness Among Youth Coming to Psychiatry Out-Patient Department of District Headquarter Hospital (DHQ), Sargodha
322 Journal of Rawalpindi Medical College (JRMC); 2020; 24(4): 322-327 Original Article Prevalence and severity of Depressive illness among youth coming to Psychiatry Out-patient department of District Headquarter Hospital (DHQ), Sargodha Rida Dawood1, Aftab Nazir2, Muhammad Shahid Javed3, Kashif Rauf4, Zunera Tanveer5, Shoaib Ahmad Malik6 1 Women Medical Officer, Children Hospital & 4 Demonstrator, Department of Biochemistry, Institute of Child Health, Faisalabad. Rawalpindi Medical University, Rawalpindi. 2 Assistant Professor, Department of Community 5 Lecturer, Department of Physiology, Medicine, Independent Medical College, Faisalabad. Bolan University of Medical and Health Sciences, Quetta. 3 Professor, Department of Physiology, 6 Assistant Professor, Department of Biochemistry, Sargodha Medical College, Sargodha. Sargodha Medical College, Sargodha. Author’s Contribution Corresponding Author Article Processing 6 Conception of study Dr. Shoaib Ahmad Malik, Received: 20/03/2020 1,3,6 Experimentation/Study conduction Assistant Professor, Accepted: 20/10/2020 1,2,4,5 Analysis/Interpretation/Discussion Department of Biochemistry, 1,2,6 Manuscript Writing Sargodha Medical College, 3 Facilitation and Material analysis Sargodha Email: [email protected] Cite this Article: Dawood, R., Nazir, A., Javed, M.S., Conflict of Interest: Nil Access Online: Rauf, K., Tanveer, Z., Malik, S.A. Prevalence and Funding Source: Nil severity of Depressive illness among youth coming to Psychiatry Out-patient department of District Headquarter Hospital (DHQ), Sargodha. Journal of Rawalpindi Medical College. 30 Dec. 2020; 24(4): 322- 327. DOI: https://doi.org/10.37939/jrmc.v24i4.1353 Abstract Introduction: Prevalence and severity of depressive illness among the youth are on the rise. Objective: To examine the prevalence and severity of depressive illness among youth coming to the psychiatry outpatient department of District Headquarters Hospital, Sargodha. -

Depression Among Medical Students of a Public Sector Medical
Annals of Clinical Obstetrics and Gynecology ISSN: 2694-491X Research Article Depression among Medical Students of a Public Sector Medical University in Pakistan Taimoor Akram Khan1, Hamna Arif2, Saba Sabahat3, Gohar Khan4 and Amjad Khan5* 1Department of Community Medicine, King Edward Medical University, Lahore, Pakistan 2Department of Gynaecology and Obstretics, Lady Aitchison Hospital, Lahore, Pakistan 3Tehsil Head Quarter Hospital, Pakistan 4Department of Medicine, Jinnah Hospital Lahore, Pakistan 5Department of Community Medicine, King Edward Medical University, Lahore, Pakistan Abstract Medical students have several risk factors for depression like lack of sleep, heavy work burden and loads of competition that puts them under enormous pressure. Objective: The objective of this study was to study the burden and severity of depression in medical students and the factors that contribute towards it. Methods: This comparative cross-sectional study was conducted at a public sector medical university in Pakistan in February 2018. Study subjects were selected using stratified random sampling. Burn’s depression checklist, an internationally validated tool for depression, was used for identification of depression and assessment of severity of symptoms. Results: Out of 300 medical students who were included in the study, 124 (41.3%) were males and 176 (58.7%) were females. 61 (20.3%) subjects were from 1st year MBBS, 61 (20.3%) from 2nd year, 65 (21.6%) from 3rd year, 61 (20.3%) from 4th year, and 52 (17.3%) from final year. The ages of subjects were between 17 Years to 26 years with mean age of 22. Out of 300 medical students, 12 students (4%) did not have depression at all, 20 students (6.7%) did not have depression but were unhappy, 102 students (34%) had mild depression whereas 117 students (39%) had moderate depression, 39 students (13%) had severe depression whereas extreme depression was seen in 10 students (3.3%). -

Vol 18 Issue 03
JAIMC PATRON Prof. Arif Tajammul The Journal of Allama Iqbal Medical College Principal Allama Iqbal Medical College/ July - September 2020, Volume 18, Issue 03 Jinnah Hospital Editorial i CHIEF EDITOR Coronaconomy – Health Verses Economy In The Time Of Corona iii Dr. Rubina Aslam Muhamamd Imran Head of Department of Psychiatry Original Articles i ASSOCIATE EDITORS Sub-Conjunctival Versus Intracameral Dexamethasone Injection Afteri 334 Sana Iftikhar Phacoemmulsifiction, A Comparative Study About Immediate Mehwish Akhtar Anterior Uveitis Sabeen Irshad Fiza Azhar, Shamshad Ali, Saqib Siddiq, Muhammad Moeen Bhatti, Ather Touseef, M. Abrar Ahmad, Khalid Waheed MANAGING EDITOR Muhammad Imran Leading Causes of Morbidities in Children under Five Years Age: 339 Knowledge and Practices Regarding Risk Factors in CMH Lahore STATISTICAL EDITORS Tahira Raza, M Asharaf Chaudhry, Ahsan Masud, Hira Kalsoom, Maheen Omer, Mamoon Akbar Qureshi Khawaja Rafay Ghazanfar, Inoshia Inam, Hamza Alvi, Jannat Sardar Sheikh Zarabia Pervaiz Butt The Prevalence of Dry Eye Disease Among Professional Computer Users 344 M. Nausherwan Adil, M. Abrar Ahmad, Usama Javaid,M.Rashid Yaseen, Ather PHOTOGRAPHY & GRAPHICS Touseef, Muhammad Moeen Bhatti Syed Ali Hassan Rizvi Knowledge, Attitudes and Practices of Hospital Waste Management amongst 349 the Paramedics of a Teaching Hospital in Lahore DESIGNING & COMPOSING Khawar Abbas Chaudhry, Amina Yousaf, Mujtaba Hasan Siddiqui, Muhammad Talal Publishers Ismaeel Tariq, Surya Fazal Hashmi, Muhammad Azhar Shah I.T MANAGER Awareness and Knowledge of PAP Smear as a Screening Test for Cervical 355 Muhamamd Shujat Ali Cancer Among Women Attending Gynaecological OPD of Services Hospital Madeeha Rashid, Kiren Khurshid Malik, Asma Mushtaq, Rubina Sohail INTERNATIONAL ADVISORY BOARD The Impact of Pterygium Surgical Excision on Corneal Astigmatism 361 Shoaib Khan (Finland) M. -

COMPREHENSIVE GERIATRIC CARE for PATIENTS with HIP FRACTURES: a REVIEW STUDY 1Dr
IAJPS 2019, 06 (10), 13456-13460 Mahnoor Aslam et al ISSN 2349-7750 CODEN [USA]: IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES Available online at: http://www.iajps.com Review Article COMPREHENSIVE GERIATRIC CARE FOR PATIENTS WITH HIP FRACTURES: A REVIEW STUDY 1Dr. Mahnoor Aslam,2Dr Shahzaib Haider,3Dr Bushra Sulaiman 1MBBS;Sargodha Medical College,Sargodha., 2MBBS;Nawaz Sharif Medical College,Gujrat., 3MBBS;Khawaja Muhammad Safdar Medical College,Sialkot. Article Received: August 2019 Accepted: September 2019 Published: October 2019 Abstract: Osteoporotic hip fractures lead to significant mortality and morbidity particularly in the vulnerable elderly population. Ortho-geriatric care, defined as collaborative care for older patients with orthopedic disorders involving orthopedic services and medical programs catering for older people, has been shown to improve outcomes. Osteoporosis and falls remain the most significant risk factors associated with hip fractures. Achieving an accurate diagnosis as soon as possible is very important when a hip fracture is being considered. The restoration of mobility and functional independence are perhaps the important goals of hip fracture surgery. Corresponding author: Dr. Mahnoor Aslam, QR code MBBS;Sargodha Medical College,Sargodha. Please cite this article in press Mahnoor Aslam et al., Comprehensive Geriatric Care for Patients with Hip Fractures: A Review Study., Indo Am. J. P. Sci, 2019; 06(10). www.iajps.com Page 13456 IAJPS 2019, 06 (10), 13456-13460 Mahnoor Aslam et al ISSN 2349-7750 INTRODUCTION: The concept of orthogeriatric care, structured Patients admitted with a hip fracture are usually orthopedic-geriatric cooperation, is not new. In elderly and they have clinical, cognitive and social United Kingdom (UK), orthopedic surgeons and problems, hence they benefit of involving a specialist geriatricians have collaborated for many years on the geriatric team during their stay in the orthopedics treatment of older people with low-energy fractures. -

College Inspection Report
PAKISTAN MEDICAL COMMISSION LAST RECOGNIZED INSPECTION GRADES OF PRIVATE MEDICAL COLLEGES Sr. Date of Previous Name of Institute City Grade No. Inspection 1. Abbottabad International Medical College Abbottabad. 17-12-2019 A 2. Abwa Medical College Faisalabad 20-11-2018 B 3. Aga Khan University Medical College Karachi. 05-08-2019 A+ 4. Akhtar Saeed Medical & Dental College Lahore. 26-08-2019 A 5. Al Aleem Medical College Lahore 29-08-2019 B 6. Al-Nafees Medical College Islamabad. 30-12-2015 C 7. Al-Tibri Medical College Karachi. 08-11-2013 B 8. Amna Inayat Medical College Lahore. 28-09-2017 C 9. Avicenna Medical College Lahore. 28-08-2019 A 10. Aziz Fatimah Medical & Dental College Faisalabad. 21-03-2018 C 11. Azra Naheed Medical College Lahore. 30-04-2015 B 12. Bahria University Medical College Karachi. 07-08-2019 B 13. Bakhtawar Amin Medical & Dental College Multan 20-02-2016 A 14. Baqai Medical College Karachi. 19-12-2018 F 15. Central Parks Medical College Lahore. 10-02-2015 C 16. CMH Institute of Medical Sciences Bahawalpur Bahawalpur 22-08-2019 C 17. CMH Kharian Medical College Kharian Cantt. 31-07-2019 B 18. CMH Lahore Medical College Lahore Cantt. 30-08-2019 A+ 19. CMH Multan Institute of Medical Sciences CIMS Multan Cantt 20-08-2019 A 20. Continental Medical College Lahore. 16-10-2018 C GRADING CRITERIA: 92.5% or above = A+, 85% or above = A, 77.5% or above = B, 70% or above = C, 69.9% or lower = F Sr. Date of Previous Name of Institute City Grade No. -

Prevalence of Neck Pain and Its Different Associated Factors Among Undergraduate Students of Sargodha Medical College
Research Article J Yoga & Physio Volume 8 Issue 1 - September 2019 Copyright © All rights are reserved by Muhammad Sajid Paracha DOI: 10.19080/JYP.2019.08.555731 Prevalence of Neck Pain and Its Different Associated Factors Among Undergraduate Students of Sargodha Medical College Muhammad Sajid Paracha1*, Breera Amjad2, Kanza Masood3, Mushyyaida Iqbal4, Seemab Mughal5, Rabbia Naseer6, Hafiz Muhammad Junaid Hassan7 and Asif Ali Butt8 1Assistant Professor, Isra University, Islamabad Campus 2Senior Lecturer, Riphah International University, Faisalabad 3Lecturer, University of Lahore, Sargodha Campus 4HOD, Sargodha Institute of Health Sciences 5Lecturer, University of Balochistan 6Lecturer, University of Balochistan 7CEO, Healthcare Physiotherapy sports, Spine Rehabilitation Center, Faisalabad 8Associate Professor, Riphah International University, Faisalabad Submission: September 11, 2019; Published: September 24, 2019 *Corresponding author: Muhammad Sajid Paracha, Assistant Professor, Isra University, Islamabad Campus Abstract Aim of Study: To find the prevalence of neck pain variation among undergraduate medical students of different academic years also exploringStudy association Design: Observational of different study Study posture, study hours and different mode of study with neck pain among undergraduate medical students. Methodology: The study was conducted in Sargodha Medical College. 500 undergraduate students fulfilling inclusion and exclusion criteria usingResults: self-created questionnaire (approved by University of Sargodha) to observe prevalence of self-reported neck pain symptoms & disability. This study concluded that out of 200 undergraduate medical students of Sargodha medical college 38% had neck pain. Out of these students 64.5% had acute neck pain while 35.5% had chronic neck pain, more prevalent in female students than in males having a percentage of 67% in females and 33% in males. -

Vol 19 Issue 02.Pdf
JAIMC PATRON Prof. Arif Tajammul The Journal of Allama Iqbal Medical College Principal Allama Iqbal Medical College/ April - June 2021, Volume 19, Issue 02 Jinnah Hospital Editorial i CHIEF EDITOR Kidney Diseases Rising in Pakistan iii Dr. Rubina Aslam Muhammad Imran Head of Department of Psychiatry Original Articles i ASSOCIATE EDITORS Sana Iftikhar Diagnostic Accuracy of Transrectal Power Doppler Ultrasound in Diagnosing 253 Mehwish Akhtar Prostate Cancers Sabeen Irshad Naeem Ahmad Khan, Tanweer Ahmad, Adnan Ahmad Sattar, Irfan Masood, Arslan Masood Fatima Iqbal, Javed Iqbal, Basma Khan, Aamer Nadeem Chaudhary Frequency of Diabetes Mellitus and Impaired Fasting Glucose in Patients with 260 MANAGING EDITOR Lichen Planus Muhammad Imran Aisha Chaudhry, Lamees Mahmood Malik, Zartaj Liaqat, Ambreen Ashraf, Amna Rasul, Maheen Irfan STATISTICAL EDITORS Mamoon Akbar Qureshi Comparison of Functional Outcome of Unipolar Versus Bipolar Uncemented 264 Zarabia Pervaiz Butt Hemiarthroplasty in Patients with Displaced Intracapsular Femoral Neck Fractures PHOTOGRAPHY & GRAPHICS Aamir Shabab, Muhammad Zubair Farooq, Muhammad Imran, Waqar Ahmed Syed Ali Hassan Rizvi Siddiqui, Fatima Arshad, Zahid Akhtar Role of Vitamin-D Along with Pregabalin for the Treatment of Painful 269 DESIGN & PRINTING Diabetic Neuropathy Talal Publishers Mohsin Masud, Lala Rukh Bangash, Shaheer Nayyar, Faridah Suhail, Muhammad [email protected] Rizwan Ahmed, Anam Fatima Bangash Association of Mean Serum Magnesium Levels in Patients Having Non-Insulin 273 INTERNATIONAL ADVISORY -

Distribution of Mbbs/Bds Seats
DISTRIBUTION OF MBBS/BDS SEATS MBBS SEATS IN MEDICAL INSTITUTIONS OF THE PUNJAB FJMU Sr. Category KEMU AIMC NMU (only SIMS FMU RMU QAMC SZMC SMC NSMC SLMC GMC GKMC KMSMC AMC SKZ Total No. MDC females) 1. Open Merit 302 301 280 206 191 287 297 273 126 79 90 100 100 100 100 100 90 3022 2. Reserved i. Disabled students 2 2 2 2 2 2 2 2 2 1 - - - - - - - 19 ii. Cholistan** - - - - - - - - 1 - - - - - - - - 01 iii. Underdeveloped districts *** - - 18 - - 10 - 6 15 - - - - - - - - 49 iv. FATA **** 1 1 1 1 - 1 1 1 - - - - - - - - - 07 Federal Government Share in v. - - - 47 - - - - - - - - - - - - - 47 FJMU* vi. Azad Jammu & Kashmir 1 1 4 13 2 4 5 4 2 - - - - - - - - 36 vii. Northern Areas(Gilgit-Baltistan) 8 8 1 4 3 2 9 18 2 - - - - - - - - 55 Foreign Students/Dual Nationality Holders under Pakistan Technical viii. 5 5 10 21 - 10 10 11 - - - - - - - - - 72 Assistance Programme***** (PTAP) Children of Overseas Pakistanis ix. and Dual Nationality Holders of 4 4 4 4 2 4 4 4 2 20 10 - - - - - 10 72 Pakistani Origin Reciprocal Seats x. - 1 3 - - - - 1 - - - - - - - - - 05 (for candidates of other provinces) xi. Goodwill Seats for Baluchistan 2 2 2 2 - 5 2 5 - - - - - - - - - 20 Grand Total 325 325 325 300 200 325 330 325 150 100 100 100 100 100 100 100 100 3405 Sr. Category DCD NID DSFMU Total No. 1. Open Merit 71 54 50 175 2. Reserved i. Disabled students 1 - - 1 Underdeveloped ii. 6 6 - 12 districts *** iii. FATA **** 1 2 - 3 Azad Jammu & iv. 3 - - 3 Kashmir Northern Areas(Gilgit- v. -
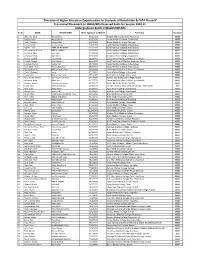
Balochistan & FATA Phase-II" Provisional Placement on MBBS/BDS Reserved Seats for Session 2020-21 Undergraduate Batch-V (BALOCHISTAN)
"Provision of Higher Education Opportunities for Students of Balochistan & FATA Phase-II" Provisional Placement on MBBS/BDS Reserved Seats for Session 2020-21 Undergraduate Batch-V (BALOCHISTAN) Sr. No NAME FATHER NAME Merit Aggregate w/MDCAT Placement Discipline 1 Wasiq Ali Tariq Tariq Bashir 93.627273 Khyber Medical College, Peshawar. MBBS 2 Rimsha Khan Nasrullah Jan 93.613636 Army Medical College, Rawalpindi. MBBS 3 Abdul Qayyum Naeem khan 93.536364 Khyber Medical College, Peshawar. MBBS 4 Asmat Ullah shams ul haq 92.845455 Ayub Medical College, Abbottabad. MBBS 5 Najeeb Ullah AMIR MUHAMMAD 92.272727 Ayub Medical College, Abbottabad. MBBS 6 Muhammad Ibrahim ABDUL JABBAR 91.650000 Ayub Medical College, Abbottabad. MBBS 7 Mohabat Khan Sado khan 91.013636 Ayub Medical College, Abbottabad. MBBS 8 Arfa Ayaz Khan Ayaz Khan 90.581818 Army Medical College, Rawalpindi. MBBS 9 Ayesha Baloch Ejaz Ahmed 90.145455 Gujranwala Medical College, Gujranwala. MBBS 10 Farooq Ahmed Altaf Hussain 89.022727 KUST Institute of Medical Sciences, Kohat. MBBS 11 Sundeep Kumar Vadoo Mal 88.740909 Ayub Medical College, Abbottabad. MBBS 12 Sana Ullah Khan Raz Muhammad 88.254545 Ayub Medical College, Abbottabad. MBBS 13 Muhammad Ali Muhammad Safdar 88.181818 Gujranwala Medical College, Gujranwala. MBBS 14 Abdul Waheed abdul 87.159091 Ayub Medical College, Abbottabad. MBBS 15 Tariq Iqbal Muhammad iqbal 85.518182 Ayub Medical College, Abbottabad. MBBS 16 Syed Haider Ali Shah Syed Asghar Ali Shah 84.440909 Nawaz Sharif Medical College, Gujrat. MBBS 17 Muheem Khan Mian dad 84.277273 Gujranwala Medical College, Gujranwala. MBBS 18 Alveena Azeem Muhammad Azeem 83.704545 Bolan Medical College, Quetta. -

Federal Capital, Islamabad Punjab Province
APPROVED PUBLIC SECTOR UNIVERSITIES / COLLEGES & THEIR CAMPUSES* Federal Capital, Islamabad Sr. # Universities / Colleges Designated Branches 1 Air University, Islamabad. Foreign Office Branch Islamabad 2 Bahria University, Islamabad. Foreign Office Branch Islamabad 3 COMSATS Institute of Information Technology, Islamabad. Foreign Office Branch Islamabad 4 Federal Urdu University of Arts, Sci. & Tech., Islamabad. Foreign Office Branch Islamabad 5 International Islamic University, Islamabad Foreign Office Branch Islamabad 6 National University of Medical Sciences, Islamabad Foreign Office Branch Islamabad 7 National University of Modern Languages, Islamabad. Foreign Office Branch Islamabad 8 National University of Science & Technology, Islamabad Foreign Office Branch Islamabad 9 National Defence University, Islamabad Foreign Office Branch Islamabad 10 Pakistan Institute of Engineering & Applied Sciences, Islamabad Foreign Office Branch Islamabad 11 Pakistan Institute of Development Economics (PIDE) Islamabad Foreign Office Branch Islamabad 12 Quaid-e-Azam University, Islamabad Foreign Office Branch Islamabad 13 Institute of Space Technology, Islamabad. Foreign Office Branch Islamabad 14 Shaheed Zulfiqar Ali Bhutto Medical University, Islamabad Foreign Office Branch Islamabad Punjab Province 1 Allama Iqbal Medical College, Lahore Main Branch Lahore. 2 Fatima Jinnah Medical College for Women, Lahore Main Branch Lahore. 3 Government College University, Lahore Main Branch Lahore. 4 King Edward Medical College, Lahore Main Branch Lahore. 5 Kinnaird College for Women, Lahore Main Branch Lahore. 6 Lahore College for Women University, Lahore. Main Branch Lahore. 7 National College of Arts, Lahore. Main Branch Lahore. 8 University of Education, Lahore. Main Branch Lahore. 9 University of Health Sciences, Lahore Main Branch Lahore. 10 University of Veterinary and Animal Sciences, Lahore. Main Branch Lahore. Sr. # Universities / Colleges Designated Branches 11 Virtual University of Pakistan, Lahore. -

PUNJAB – Public Sector Medical Colleges PUNJAB – Private Sector
PUNJAB – Public Sector Medical Colleges S.No. Medical Colleges 1. Allama Iqbal Medical College, Lahore. Recognized. Being re-inspected for seat allocation. 2. Army Medical College, Rawalpindi. Recognized. Allowed to admit 150 MBBS students per year. 3. Fatima Jinnah Medical College for Women, Recognized. Allowed to admit 250 MBBS students Lahore per year. 4. King Edward Medical College, Lahore. Recognized. Being re-inspected for seat allocation. 5. Nishtar Medical College, Multan Recognized. Allowed to admit 250 MBBS students per year. 6. Punjab Medical College, Faisalabad. Recognized. Being re-inspected for seat allocation. 7. Quaid-e-Azam Medical College, Bahawalpur. Recognized. Allowed to admit 300 MBBS students per year. 8. Rawalpindi Medical College, Rawalpindi. Being re-inspected for seat allocation. Recognized. 9. Services Institute of Medical Sciences, Allowed to admit 150 MBBS students per year. Lahore. Recognized. 10. Sargodha Medical College, Sargodha. Allowed to admit 100 MBBS students per year. Recognized. 11. Shaikh Zayed Medical College, Rahim Yar Recognized. Allowed to admit 100 MBBS students Khan. per year. PUNJAB – Private Sector Medical Colleges S.No. Medical Colleges 12. FMH College of Medicine & Dentistry, Lahore Allowed to admit 100 MBBS students per year. Recognized. 13. Foundation University Medical College, Recognized. Allowed to admit 100 MBBS students Rawalpindi per year. 14. Islamic International Medical College, Recognized. Allowed to admit 100 MBBS students Rawalpindi. per year. 15. Lahore Medical & Dental College, Lahore . Recognized.Allowed to admit 100 MBBS students per year. 16. Shifa College of Medicine, Islamabad. Recognized. Allowed to admit 100 MBBS students per year. 17. Wah Medical College, Wah Cantt. Recognized. Allowed to admit 100 MBBS students per year.